11/11/2020
Inhibition de l'histone déacetylsae pour l'hémorragie
Histone deacetylase 6 inhibition improves survival in a swine model of lethal hemorrhage, polytrauma, and bacteremia
Biesterveld BE et Al. J Trauma Acute Care Surg . 2020 Nov;89(5):932-939.
---------------------
Le TXA fait partie de l'arsenal thérapeutique en cas d'hémorragie sévère d'origine traumatique. Cela pourrait être également le cas des inhibiteurs de l'histone déacétylsae, famille de l'acide valproïque - Dépakine-
---------------------
Background: Trauma is the leading cause of death for young Americans. Nonspecific histone deacetylase inhibitors, such as valproic acid, have been shown to improve survival in preclinical models of lethal trauma, hemorrhage, and sepsis. The doses needed to achieve a survival benefit are higher than Food and Drug Administration-approved doses, and the nonspecificity raises concerns about unintended adverse effects. The isoform-specific histone deacetylase 6 inhibitor, ACY-1083, has been found to be as efficacious as valproic acid in a rodent model of hemorrhagic shock. We hypothesized that ACY-1083 treatment would improve survival in a swine model of lethal hemorrhage, polytrauma, and bacteremia.
Methods: Swine were subjected to 45% blood volume hemorrhage, brain injury, femur fracture, rectus crush, splenic and liver lacerations, and colon injury. After 1 hour of shock (mean arterial pressure, 30-35 mm Hg), animals were randomized to normal saline resuscitation (control) or normal saline plus ACY-1083 30 mg/kg treatment (n = 5/group). After 3 hours (simulating delayed evacuation), packed red blood cells and antibiotics were administered, the colon injury was repaired, and the abdomen was closed. Animals were then monitored for another 4 hours. Survival was assessed using Kaplan-Meier and log-rank test.
Results: This combination of injuries was lethal. All animals became bacteremic, in addition to the severe hemorrhagic shock. Survival in the control group was 0%, and ACY-1083 treatment increased survival to 80% (p = 0.019). There was no difference in the brain lesion size between the groups.
Conclusion: A single dose of ACY-1083 markedly improves survival in an otherwise lethal model of polytrauma, hemorrhagic shock, and bacteremia.
Plasma préhospitalier. Surtout pour certains
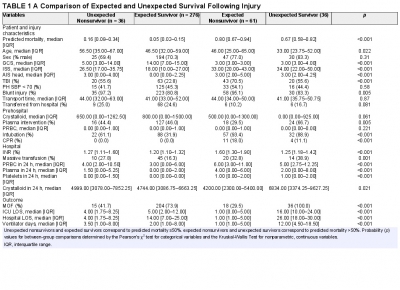
Characterization of unexpected survivors following a prehospital plasma randomized trial
J Trauma Acute Care Surg. 2020 Nov;89(5):908-914
Background: Prehospital plasma improves survival for severely injured trauma patients transported by air ambulance. We sought to characterize the unexpected survivors, patients who survived despite having high predicted mortality after traumatic injury.
Methods: The Prehospital Air Medical Plasma trial randomized severely injured patients (n = 501) to receive either standard care (crystalloid) or two units of prehospital plasma followed by standard care fluid resuscitation. We built a generalized linear model to estimate patient mortality. Area under the receiver operating characteristic curve was used to evaluate model performance. We defined unexpected survivors as patients who had a predicted mortality greater than 50% and survived to 30 days. We characterized patient demographics, clinical features, and outcomes of the unexpected survivors. Observed to expected (O/E) ratios and Z-statistics were calculated using model-estimated mortality for each cohort.
Results: Our model predicted mortality better than Injury Severity Score or Revised Trauma Score parameters and identified 36 unexpected survivors. Compared with expected survivors, unexpected survivors were younger (33 years [24, 52 years] vs. 47 years [32, 59 years], p = 0.013), were more severely injured (Injury Severity Score 34 [22, 50] vs. 18 [10, 27], p < 0.001), had worse organ dysfunction and hospital resource outcomes (multiple organ failure, intensive care unit, hospital length of stay, and ventilator days), and were more likely to receive prehospital plasma (67 vs. 46%, p = 0.031).
Nonsurvivors with high predicted mortality were more likely to receive standard care resuscitation (p < 0.001). Unexpected survivors who received prehospital plasma had a lower observed to expected mortality than those that received standard care resuscitation (O/E 0.56 [0.33-0.84] vs. 1.0 [0.73-1.32]). The number of prehospital plasma survivors (24) exceeded the number of predicted survivors (n = 10) estimated by our model (p < 0.001).
Conclusion: Prehospital plasma is associated with an increase in the number of unexpected survivors following severe traumatic injury. Prehospital interventions may improve the probability of survival for injured patients with high predicted mortality based on early injury characteristics, vital signs, and resuscitation measures.
22/10/2020
Albumine: Le retour ?
Should Albumin be Considered for Prehospital Resuscitation in Austere Environments? A Prospective Randomized Survival Study in Rabbits
Kheirabadi BS et Al. Shock . 2020 Sep;54(3):358-367.
--------------------------------
Le remplissage vasculaire par albumine diluée n'est pas spécialement recommandé. Pourtant il semblerait que cela soit à tort dans certains environnements. C'est du moins ce que laisse penser ce travail expérimental chez le lapin.
--------------------------------
Background:
The new guidelines for prehospital care of combat casualties in shock recommend administration of whole blood or blood components to increase blood pressure to a permissible hypotensive level (i.e., hypotensive resuscitation [HR]). We investigated if 2 h of HR using limited volumes of whole blood, plasma, or albumin would lead to full recovery and long-term survival of rabbits subjected to severe hemorrhagic shock (HS).
Methods:
Following instrumentation, laparotomy was performed on IV-anesthetized spontaneously breathing New Zealand white rabbits (3.0 kg -3.5 kg). Next, ∼40% of rabbits' blood volume was removed producing HS (mean arterial pressure [MAP]∼20 mm Hg). Fifteen minutes later, rabbits were resuscitated with a limited volume (12.5 mL/kg) of rabbit whole blood (fresh whole blood [FWB]), rabbit fresh frozen plasma (FFP), or 5% human albumin (ALB) to a target pressure (MAP) of 60 mm Hg (n=8/grp) and monitored for 2 h. Liver bleeding time was measured at baseline and 10 min after HR. Subsequently, animals were fully resuscitated (blood + lactated Ringer [LR]), surgically repaired, and recovered for 8 days. An untreated group (n = 6) was also included.
Results:
Following HS, lactate and base deficit levels were increased to 8.2 ± 1.6 and 12.9 ± 3.1 mM respectively with no difference among groups. A lower volume of FWB volume was required to reach the target MAP (P < 0.05 vs. ALB) but MAP declined during the HR period (P < 0.01 vs. ALB).
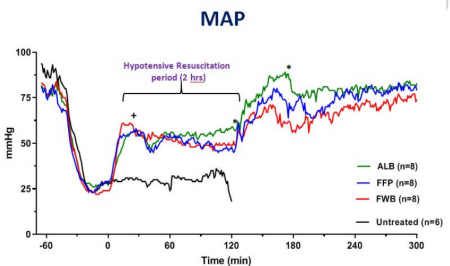
FWB provided higher hematocrit and platelets but it did not reduce lactate level faster than other fluids. Beside higher fibrinogen, no differences were found in hemostatic or resuscitative effects of FFP versus ALB. Bleeding time was prolonged with ALB and FFP fluids but unchanged with FWB. Untreated rabbits died during shock or shortly after. All treated rabbits except one recovered and lived for 8 days with normal blood tests and similar tissue histology.
Conclusions:
Two hours of HR using a limited volume of FWB, FFP, or ALB led to full recovery and long-term survival of rabbits subjected to HS. Apart from bleeding time, no clinically significant differences were found among the three fluids. Five percent human albumin solutions are isotonic, iso-oncotic, ready-to-use, stable, and compatible with all blood types and should be considered for prehospital resuscitation where blood products are not available or not accepted.
| Tags : remplissage
02/01/2020
Le fibrinogène se conserve bien au chaud comme au froid
Stability of Fibrinogen Concentrate in Human Blood Samples: An In Vitro Study
Objectives:
This study was designed to assess the stability and functional activity of fibrinogen concentrates subjected to the changes in temperature and duration observed in field conditions.
Methods:
Fibrinogen concentrate was stored at -20°C (12 vials), 22°C (12 vials), and 50°C with 80% humidity (12 vials), for up to 6 mo. At each temperature, three vials of fibrinogen concentrate were taken out at 0, 1, 3, and 6 mo and reconstituted. On analysis days, blood samples were taken from a single healthy donor to collect plasma samples. The donor plasma was mixed with commercial fibrinogen-deficient plasma to make fibrinogen-adjusted plasma (FAP). An aliquot of the reconstituted fibrinogen concentrate was used for quantification of stored fibrinogen content (using STA-R) and function (Rotem - Fibtem) in FAP.
Results:
At 22°C for 0, 1, 3, and 6 mo, there were no significant changes observed in fibrinogen content (1,223 ± 42 mg/vial, 1,286 ± 86 mg/vial, 1,234 ± 76 mg/vial, and 1,178 ± 64 mg/vial), prothrombin time (13.5 ± 0.1 s, 13.7 ± 0.6 s, 13.3 ± 0.4 s, and 13.7 ± 0.2 s), or activated partial thromboplastin time (31.1 ± 0.2 s, 32.0 ± 0.2 s, 31.5 ± 0.2 s, and 32.0 ± 0.8 s), respectively. There were also no significant changes observed in any of the Fibtem measurements. Similarly, no differences were observed in these variables over time at -20°C and 50°C with 80% humidity.
Conclusions:
Fibrinogen concentrate maintained its content and function when stored at -20°C to 50°C with up to 80% humidity for 6 mo.
| Tags : fibrinogène
04/12/2019
HEA 130: Protègerait le glycocalyx ?
The protective effect of hydroxyethyl starch solution on the glycocalyx layer in an acute hemorrhage mouse model.
- --------------------------------------------------------
Le recours au HEA pour le remplissage vasculaire est très décrié notamment à cause d'effets rénaux délétères observés tout particulièrement en cas de sepsis. Les HEA peuvent néanmoins être utilisés en cas de non restauration de l'hémodynamique après emploi de cristalloides isotoniques et en attendant les produits de transfusion (reco 6 de la RFE sur le choc hémorragique). Ce travail semble donc tempérer un peu le rejet des HEA pour la réanimation du choc hémorragique. Il montre une moindre élévation de syndecan-1 lors de l'emploi d'HEA 130 qu'après Salé.
--------------------------------------------------------
PURPOSE:
Fluid therapy focused on glycocalyx (GCX) protection in hemorrhagic shock is a current focus of research. Hydroxyethyl starch (HES) solution is commonly used for fluid resuscitation; however, its effects on the GCX remain unclear. The primary aim of this study was to explore the protective effect of HES130 in maintaining GCX thickness and reducing plasma syndecan-1 expression.
METHODS:
An acute hemorrhage murine model with the dorsal skin chambers was used to measure GCX thickness and to evaluate vascular permeability. Groups of mice were treated with normal saline (NS), albumin (NS-A), HES130 (NS-V), or no exsanguination or infusion (C). We measured syndecan-1 plasma concentrations, performed blood gas analysis, and analyzed the 7-day cumulative mortality.
RESULTS:
GCX thickness in NS mice was significantly reduced compared to that in group C, but no other groups showed a difference compared to group C. The plasma concentration of syndecan-1 was significantly higher in NS mice than in group C. There were no significant differences in the fluorescence intensity of dextran in the interstitial space. HES70 leakage was suppressed in NS-V mice compared to those in other groups. HES70 was localized to the inner vessel wall in C, NS, and NS-A mice, but not in group NS-V. Blood gas analysis indicated that pH and lactate showed the greatest improvements in NS-V mice. The 7-day cumulative mortality rate was the highest in group NS.
CONCLUSION:
Resuscitation with HES130 protected the GCX and suppressed vascular permeability of HES70 during early stages of acute massive hemorrhage.
26/11/2019
TXA: Recommandé mais pas administré
An Analysis of Adherence to Tactical Combat Casualty Care Guidelines for the Administration of Tranexamic Acid.
BACKGROUND:
Hemorrhage is the leading cause of potentially survivable deaths in combat. Previous research demonstrated that tranexamic acid (TXA) administration decreased mortality among casualties. For casualties expected to receive a transfusion, the Committee on Tactical Combat Casualty Care (TCCC) recommends TXA. Despite this, the use and adherence of TXA in the military prehospital combat setting, in accordance with TCCC guidelines, is low.
OBJECTIVES:
We sought to analyze TXA administration and use among combat casualties reasonably expected to require blood transfusion, casualties with tourniquet placement, amputations, and gunshot wounds.
METHODS:
Based on TCCC guidelines, we measured proportions of patients receiving prehospital TXA: casualties undergoing tourniquet placement, casualties sustaining amputation proximal to the phalanges, patients sustaining gunshot wounds, and patients receiving ≥10 units of blood products within 24 h of injury. Univariable and multivariable analyses were also completed.
RESULTS:
Within our dataset, 255 subjects received TXA. Four thousand seventy-one subjects had a tourniquet placed, of whom 135 (3.3%) received prehospital TXA; 1899 subjects had an amputation proximal to the digit with 106 (5.6%) receiving prehospital TXA; and 6660 subjects had a gunshot wound with 88 (1.3%) receiving prehospital TXA. Of 4246 subjects who received ≥10 units of blood products within the first 24 h, 177 (4.2%) received prehospital TXA.
CONCLUSIONS:
We identified low TXA administration despite TCCC recommendations. Future studies should seek to both identify reasons for limited TXA administration and methods to increase future utilization.
19/11/2019
PLyo à température ambiante: Pas si sûr !
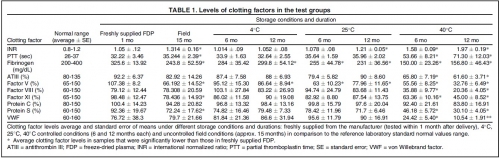
Freeze-dried plasma stability under prehospital field conditions.
BACKGROUND:
This study evaluated the effect of routine, uncontrolled, Israeli field storage conditions on the stability and efficacy of Lyo-Plas N freeze-dried plasma (FDP). We evaluated clotting factors V, VIII, and XI; proteins S and C; fibrinogen; partial thromboplastin time (PTT); antithrombin III (ATIII); von Willebrand factor (VWF); and international normalized ratio (INR) in FDP stored at 4°C, 25°C, and 40°C for 6 and 12 months, as well as FDP returned from field units after uncontrolled storage for 15 months (manufacturer's shelf life).
METHODS AND MATERIALS:
After reconstitution, clotting factor levels were compared to those of freshly supplied FDP doses.
RESULTS:
At 4°C for 12 months, factor V decreased slightly. At 25°C, average fibrinogen and factor V content were significantly lower at both periods, and INR was higher after 12 months. At 40°C, all samples were out of normal range in at least one clotting factor after 6 or 12 months. After field storage for 15 months, fibrinogen, factors V and XI, PTT, and protein S were significantly decreased, and INR increased.
However, these levels were still within laboratory norms. Statistically significant difference in clotting factors compared to laboratory normal range was found in INR (higher) and factor V (lower).
CONCLUSIONS:
Our data show minimal decreases in clotting factors in FDP after storage under field conditions, when compared to laboratory normal ranges. Along with the many advantages of FDP, this supports its use at the point of injury under battlefield conditions, despite uncontrolled storage environments. Under controlled storage conditions at 4°C, shelf life could possibly be extended, although further study is required.
25/07/2019
PGR + Plasma pour une meilleure survie long terme
Is prehospital blood transfusion effective and safe in haemorrhagic trauma patients? A systematic review and meta-analysis.
BACKGROUND:
Life-threatening haemorrhage accounts for 40% mortality in trauma patients worldwide. After bleeding control is achieved, circulating volume must be restored. Early in-hospital transfusion of blood components is already proven effective, but the scientific proof for the effectiveness of prehospital blood-component transfusion (PHBT) in trauma patients is still unclear.
OBJECTIVE:
To systematically review the evidence for effectiveness and safety of PHBT to haemorrhagic trauma patients.
METHODS:
CINAHL, Cochrane, EMBASE, and Pubmed were searched in the period from 1988 until August 1, 2018. Meta-analysis was performed for matched trauma patients receiving PHBT with the primary outcomes 24-hour mortality and long-term mortality. Secondary outcome measure was adverse events as a result of PHBT.
RESULTS:
Trauma patients who received PHBT with simultaneous use of packed red blood cells (pRBCs) and plasma showed a statistically significant reduction in long-term mortality (OR = 0.51; 95% CI, 0.36-0.71; P < 0.0001) but no difference in 24-hour mortality (OR = 0.47, 95% CI, 0.17-1.34; P = 0.16). PHBT with individual use of pRBCs showed no difference in long-term mortality (OR = 1.18; 95% CI, 0.93-1.49; P = 0.17) or 24-hour mortality (OR = 0.92; 95% CI, 0.46-1.85; P = 0.82).
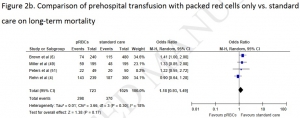
In a total of 1341 patients who received PHBT, 14 adverse events were reported 1.04%, 95% CI 0.57-1.75%.
CONCLUSIONS:
PHBT with simultaneous use of both pRBCs and plasma resulted in a significant reduction in the odds for long-term mortality. However, based on mainly poor quality evidence no hard conclusion can be drawn about a possible survival benefit for haemorrhagic trauma patients receiving PHBT. Overall, PHBT is safe but results of currently ongoing randomised controlled trials have to be awaited to demonstrate a survival benefit.
19/05/2019
Frères de sang, petite histoire de la transfusion
Émile Jeanbrau, Georges Dehelly, Maurice Guillot, Albert Hustin, Bruce et Oswald Robertson
Jean Baptiste Denis (1635 - 1704) réalise la première transfusion de sang chez l'Homme le 15 juin 1667. Le patient est un jeune homme de 15-16 ans, atteint de fièvre depuis deux mois, et déjà traité par plus de 20 saignées ! Il présente une perte de mémoire et une incapacité à produire le moindre effort, signes attribués par Denis à l'effet des saignées. Le traitement transfusionnel consiste en fait en l'échange de 3 onces (environ 100 mL) de sang du patient contre 9 onces (environ 300 mL) de sang de mouton. Le suivi à court terme montre une amélioration clinique très rapide, avec reprise de l'activité.
C'est le 27 mars 1914 qu'est réussie dans l'Histoire la première transfusion sanguine par poche, réalisée en BELGIQUE par Albert HUSTIN sur un patient anémié par des hémorragies coliques de longue durée. Au PAYS BASQUE, le 16 octobre 1914 a eu lieu, à l'Hôpital de BIARRITZ, la première transfusion sanguine directe de la première Guerre Mondiale : Isidore COLAS, un soldat breton (né à BANNALEC) en convalescence à la suite d'une blessure à la jambe, sauve par le don de son sang le Caporal Henri LEGRAIN (origine de LAON dans l'Aisne) du 45ème d'Infanterie, arrivé exsangue du Front. Leurs sangs devaient être compatibles puisque l'opération réussit. "Je le vis peu à peu se recolore et renaître à la vie" expliqua un des médecins

"On a rapporté récemment un cas de transfusion effectué avec plein de succès à Montpellier par le docter Jeanbrau et le professeur Hedon. Notre photographe représente les deux frères d'armes, devenus frères de sang, vingt-cinq jours après la transfusion qui sauva l'un grâce au sacrifice de l'autre: à gauche, le soldat réserviste Créchet, du 68e de ligne, amputé après une terrible hémorragie; à droite, le "donneur", Emile Barthélémy, du 81e de ligne, légèrement blessé à Gerbeviller" source
Tout ceci a été rendu possible dans les armées françaises grâce à l'investissement du Pr Jeanbrau, pionner de la transfusion en France.
Natif d’Alès, il étudie la médecine à Montpellier, jusqu’au doctorat en 1898 ; il devient chirurgien et s’oriente vers l’urologie. En 1914 il est mobilisé comme chirurgien à l’hôpital d’évacuation de Biarritz. Le 16 octobre, il transfuse parla technique de la canule d’Elsberg le soldat Henri Legrain, blessé le 28 septembre, amputé de la cuisse droite. Le donneur est un éclopé, le soldat Isidore Colas (on appelait “éclopé”, dans le langage de la médecine militaire, un blessé léger, convalescent). Henri Legrain guérit ; il mourut en 1987, à l’âge de 97 ans ! Émile Jeanbrau pratiqua quelques autres transfusions à l’aide de la canule d’Elsberg.
Mais, jugeant l’opération “trop difficile, trop minutieuse et trop longue pour entrer dans la pratique d’urgence”, il passa au tube de Kimpton-Brown paraffiné, qu’il améliora progressivement dans les années suivantes (modifications de forme, de volume, du système d’aspiration et d’insufflation, introduction de 25 à 30 ml d’une solution de citrate de sodium). Il était alors, près du front, chirurgien-chef de l’ambulance automobile chirurgicale (“Autochir”) n°13.
Du début du conflit jusqu’à la fin de 1914, on estime à 50, tout au plus, les transfusions sanguines pratiquées sur des blessés de l’armée française, et par Émile Jeanbrau, Georges Dehelly et Maurice Guillot pour la plupart.
Georges Dehelly s’était formé à la transfusion sanguine, avant-guerre, lors d’un stage de perfectionnement auprès de Crile aux États-Unis. Il fut l’auteur, avec Maurice Guillot etLouis Morel d’un d es premiers ouvrages français sur la transfusion sanguine .
Un point anecdotique de la transfusion sanguine dans la période 1914-1916 concerne un vieux général français. Au printemps de 1916, épuisé par la maladie, il démissionne de son poste de ministre de la Guerre. Le 18 mai, il est opéré de la prostate dans une clinique de Versailles. Après une hématurie massive il est transfusé, le donneur étant son chirurgien lui-même ! Il décède néanmoins, le lendemain 27 mai : Joseph Simon Gallieni (1849-1916), gouverneur militaire de Paris en août 1914, l’homme des “taxis de la Marne”, est donc très vraisemblablement le premier général de l’armée française à recevoir une transfusion sanguine. source
Lire: La transfusion sanguine pendant la Grande Guerre (1914 - 1918).
Salé 7,5%: Ne pas dépasser 500 ml
The effects of hypertonic saline solution (7.5%) on coagulation and fibrinolysis: an in vitro assessment using thromboelastography.
-----------------------------------
Ce travail met en avant le caractère dose-dépendant des effets des solutions hypertoniques salines sur la coagulation. Elle confirme des travaux plus anciens (1) et milite au moins pour une utilisation raisonnée, limitée à deux administrations. Ceci est cohérent avec la proposition faite par la procédure du sauvetage au combat.
-----------------------------------
We studied the effects of hypertonic (7.5%) and normal saline on coagulation and fibrinolysis in an in vitro model using thromboelastography of human whole blood. Reaction times increased and alpha angles decreased with hypertonic saline replacement at 7.5% blood volume compared with similar dilution with normal saline.
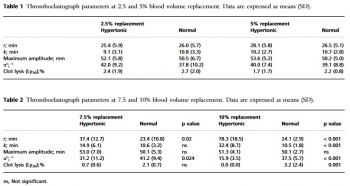
At 10% blood volume replacement with hypertonic saline, reaction and coagulation times were significantly increased and alpha angles were decreased. Clot lysis at 30 min was also significantly reduced. We conclude that 7.5% hypertonic saline solution has anticoagulant effects if it replaces 7.5% or more of blood volume.
30/04/2019
Sang total: Une vision US
Whole Blood in Trauma: A Review for Emergency Clinicians.
Comme quoi une roue est faite pour tourner. La même démarche chez les norvégiens en prégospitalier (1)
-------------------------------------------
BACKGROUND:
Blood products are a cornerstone of trauma resuscitation. From the historically distant battlefields of World War II through present-day conflict around the globe, whole blood (WB) has been a potent tool in the treatment of massive hemorrhagic shock. Component therapy with a targeted ratio of packed red blood cells, platelets, and plasma has previously been utilized.
OBJECTIVES:
This narrative review describes modern-day WB transfusion, its benefits, potential drawbacks, and implementation.
DISCUSSION:
The current form of stored low-titer O WB seems to be the safest and most effective solution. There are many advantages to WB, including the maintenance of coagulation factors, the lack of subsequent thrombocytopenia, and the reduction of infused anticoagulant. Several studies suggest its utility in trauma.
Most of the disadvantages of WB stem from a lack of prospective data on the topic, which are likely forthcoming. Logistical issues likely present the greatest barrier to this therapy, but an advanced prehospital protocol developed in San Antonio, Texas, has successfully overcome several of these challenges.
CONCLUSIONS:
Although stored WB holds promise, it is not without its distinct challenges, including logistical issues, which this article addresses. There are programs underway currently that demonstrate its feasibility in metropolitan areas. As demonstrated in military settings, WB is likely the ideal resuscitation fluid for civilian trauma in the prehospital and emergency department settings.
26/04/2019
Fibrinogène lyophilisé: Stable 6 mois jusquà 50°c
Stability of Fibrinogen Concentrate in Human Blood Samples: An In Vitro Study
OBJECTIVES:
This study was designed to assess the stability and functional activity of fibrinogen concentrates subjected to the changes in temperature and duration observed in field conditions.
METHODS:
Fibrinogen concentrate was stored at -20°C (12 vials), 22°C (12 vials), and 50°C with 80% humidity (12 vials), for up to 6 mo. At each temperature, three vials of fibrinogen concentrate were taken out at 0, 1, 3, and 6 mo and reconstituted. On analysis days, blood samples were taken from a single healthy donor to collect plasma samples. The donor plasma was mixed with commercial fibrinogen-
RESULTS:
At 22°C for 0, 1, 3, and 6 mo, there were no significant changes observed in fibrinogen content (1,223 ± 42 mg/vial, 1,286 ± 86 mg/vial, 1,234 ± 76 mg/vial, and 1,178 ± 64 mg/vial), prothrombin time (13.5 ± 0.1 s, 13.7 ± 0.6 s, 13.3 ± 0.4 s, and 13.7 ± 0.2 s), or activated partial thromboplastin time (31.1 ± 0.2 s, 32.0 ± 0.2 s, 31.5 ± 0.2 s, and 32.0 ± 0.8 s), respectively.
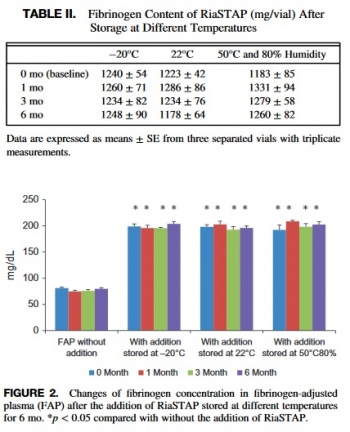
There were also no significant changes observed in any of the Fibtem measurements. Similarly, no differences were observed in these variables over time at -20°C and 50°C with 80% humidity.
CONCLUSIONS:
Fibrinogen concentrate
24/04/2019
Numéro special THOR
01/04/2019
Oxsealife: LE cristalloïde de demain ?
Blood transfusion is given according to haemoglobin thresholds aimed at restoration of arterial oxygen-carrying capacity. Patient survival after severe haemorrhagic shock depends on restoration of microvascular perfusion, tissue oxygen delivery, endothelial function and organ integrity. We investigated a novel crystalloid fluid designed for tissue oxygen delivery, Oxsealife® , with components that generate microvascular nitric oxide and scavenge reactive oxygen species generated during ischaemia-reperfusion injury. The amount of dissolved oxygen in blood progressively increased during step-wise in vitro haemodilution with this fluid, suggesting that the oxygen solubility coefficient of blood is dynamic, not static. We performed a pilot safety and efficacy study to compare resuscitation with this novel crystalloid vs. whole blood transfusion in a swine haemorrhagic shock model with animals bled to an arterial lactate oxygen debt target. Despite contributing no haemoglobin, viscosity nor oncotic potential, resuscitation with Oxsealife after severe haemorrhagic shock restored central haemodynamic parameters comparable to stored allogeneic blood transfusion. Tissue perfusion, oxygenation and metabolic outcomes were equivalent between treatment groups. Increased consumption of bicarbonate in animals given Oxsealife suggested greater capillary recruitment and enhanced clearance of acidic tissue metabolites. Serum markers of organ function, animal activity during recovery and histological analysis of tissue morphology and endothelial glycocalyx integrity confirmed functional recovery from haemorrhagic shock. We conclude that recovery of tissue oxygen delivery and organ function after haemorrhagic shock may not be dependent on treatments that increase haemoglobin levels. Oxsealife shows promise for treatment of severe haemorrhagic shock and may reduce the requirement for allogeneic blood products.
11/03/2019
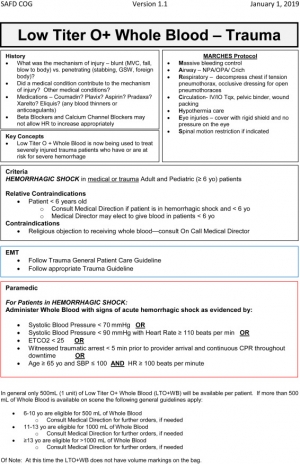
Sang total de banque: Oui mais avec une logistique adhoc
The use of whole blood in US military operations in Iraq, Syria, and Afghanistan since the introduction of low-titer Type O whole blood: feasibility, acceptability, challenges.
------------------------------
L'emploi de sang total est redevenu d'actualité, qu'il s'agisse de sang prélevé sur pied ou conservé en banque. Ce dernier est de plus en plus employé, notamment en role 2, avec cependant une difficulté qui tient à une moindre durée de conservation (21 jours en poche CPD et 35 jours en poche CPDA-1). Réduire le délai prélèvement-délivrance sur zone est donc fondamental si l'on souhaite en tirer le meilleur bénéfice. La production sur zone de combat de ce type de produit pourrait être une solution.
------------------------------
BACKGROUND:
Hemorrhage is the leading cause of preventable death in military and civilian traumatic injury. Blood product resuscitation improves survival. Low-titer Type O Whole Blood (LTOWB) was recently re-introduced to the combat theater as a universal resuscitation product for hemorrhagic shock. This study assessed the utilization patterns of LTOWB compared to warm fresh whole blood (WFWB) and blood component therapy (CT) in US Military Operations in Iraq/Syria and Afghanistan known as Operation Inherent Resolve (OIR) and Operation Freedom's Sentinel (OFS) respectively. We hypothesized LTOWB utilization would increase over time given its advantages.
STUDY DESIGN AND METHODS:
Using the Theater Medical Data Store, patients receiving blood products between January 2016 and December 2017 were identified. Product utilization ratios (PUR) for LTOWB, WFWB, and CT were compared across Area of Operations (AORs), medical treatment facilities (Role 2 vs. Role 3), and time. PUR was defined as number of blood products transfused/(number of blood products transfused + number of blood products wasted).
RESULTS:
The overall PUR for all blood products was 17.4%; the LTOWB PUR was 14.3%. Over the study period, the total number of blood products transfused increased 133%. Although the total whole blood (WB) increased from 2.1% to 6.6% of all products transfused, WFWB use remained at 2% while LTOWB transfusions increased from 0.5% to 4%. Transfusion of LTOWB occurred more in austere Role 2 facilities compared to Role 3 hospitals.
CONCLUSIONS:
LTOWB transfusion is feasible in austere, far-forward environments. Further investigation is needed regarding the safety, clinical outcomes, and drivers of LTOWB transfusions.
04/03/2019
Transfusion: Numéro spécial Trauma
22/01/2019
Thrombosomes: C'est parti
Safety evaluation of a lyophilized platelet-derived hemostatic product.
BACKGROUND:
Hemorrhage causes significant morbidity and mortality in people aged <65 years. A lyophilized platelet-derived hemostatic agent (Thrombosomes) demonstrated hemostatic efficacy in animal models. We report the results of the first safety trial of autologous Thrombosomes given to normal subjects.
STUDY DESIGN AND METHODS:
Ten subjects received autologous Thrombosomes prepared from their apheresis platelets, and five control subjects received a buffer solution. There were five cohorts, with three subjects per cohort (two in the Thrombosomes group and one in the control group). Doses escalated from 1/1,000 to 1/10 of a proposed efficacious dose. Cohorts 4 and 5 received the highest dose, but in Cohort 5, one-half the dose was infused 2 hours apart. Cohorts 1 through 3 were monitored for 42 days, Cohorts 4 and 5 were monitored for 60 days using hematology, coagulation, and chemistry assays and antibody testing.
RESULTS:
There were no serious adverse events (AEs) and no subject withdrawals. There were eight treatment-related AEs (TRAEs) in 5 of 15 subjects (33%) (four in the Thrombosomes group and one in the control group). Of four subjects receiving the highest doses, three had TRAEs. One had elevated D-dimer, prothrombin fragment 1 + 2, and white blood cell count (subject had concurrent upper respiratory tract infection); one had T-wave inversions in precordial leads V2 and V3 without elevated troponin or symptoms; and one had a platelet autoantibody without change in plateletcount. All subjects' TRAEs resolved by Day 21.
CONCLUSION:
There were no serious AEs in this small study. Thrombosomes were considered safe at the doses assessed. Future, larger trials will be needed to further assess safety and efficacy.
21/01/2019
Sang total: A nouveau disponible
L'emploi de sang total n'est pas une nouveauté. C'est même la base de la transfusion sanguine dans les pays à faible infrastructure médicale.

Nous en avions, comme le PLyo, perdu l'usage avec l'arrivée des transfusions fractionnées par composants. Le renouveau du sang total pour la prise en charge des blessés de guerre l'a remis en selle, de même que toutes les techniques de préparation permettant sa conservation tout en préservant ses qualités hémostatiques. Le sang total « froid » préparé par le CTSA est un produit universel, déleucocyté, qualifié biologiquement, à bas titre d'hémolysine. Son intérêt principal est d'apporter plaquettes fonctionnelles et facteurs de coagulation. Il reste à démontrer que les qualités potentielles de ce "nouveau" produit ne s'altèrent pas avec le temps. Il peut être a priori conservé 3 semaines (?). Il s'agit d'une nouvelle importante pour le développement de la transfusion préhospitalière.
Pas + de 10 CGR âgés de +22j pour le traumatisé grave
STUDY OBJECTIVE:
The transfusion of older packed RBCs may be harmful in critically ill patients. We seek to determine the association between packed RBC age and mortality among trauma patients requiring massive packed RBC transfusion.
METHODS:
We analyzed data from the Pragmatic, Randomized Optimal Platelet and Plasma Ratios trial. Subjects in the parent trial included critically injured adult patients admitted to 1 of 12 North American Level I trauma centers who received at least 1 unit of packed RBCs and were predicted to require massive blood transfusion. The primary exposure was volume of packed RBC units transfused during the first 24 hours of hospitalization, stratified by packed RBC age category: 0 to 7 days, 8 to 14 days, 15 to 21 days, and greater than or equal to 22 days. The primary outcome was 24-hour mortality. We evaluated the association between transfused volume of each packed RBC age category and 24-hour survival, using random-effects logistic regression, adjusting for total packed RBC volume, patient age, sex, race, mechanism of injury, Injury Severity Score, Revised TraumaScore, clinical site, and trial treatment group.
RESULTS:
The 678 patients included in the analysis received a total of 8,830 packed RBC units. One hundred patients (14.8%) died within the first 24 hours. On multivariable analysis, the number of packed RBCs greater than or equal to 22 days old was independently associated with increased 24-hour mortality (adjusted odds ratio [OR] 1.05 per packed RBC unit; 95% confidence interval [CI] 1.01 to 1.08): OR 0.97 for 0 to 7 days old (95% CI 0.88 to 1.08), OR 1.04 for 8 to 14 days old (95% CI 0.99 to 1.09), and OR 1.02 for 15 to 21 days old (95% CI 0.98 to 1.06). Results of sensitivity analyses were similar only among patients who received greater than or equal to 10 packed RBC units.
CONCLUSION:
Increasing quantities of older packed RBCs are associated with increased likelihood of 24-hour mortality in trauma patients receiving massive packed RBC transfusion (≥10 units), but not in those who receive fewer than 10 units.
16/01/2019
Plyo prehospitalier: N'améliore pas la survie ?
The impact of prehospital administration of freeze-dried plasma on casualty outcome.
BACKGROUND:
Hemorrhage is the most common preventable cause of death in both civilian and military trauma. There is no consensus regarding the appropriate fluid resuscitation protocol. Plasma, as a resuscitative fluid, has substantial benefits as a volume expander, owing to its relatively high oncotic pressure and its positive effect on trauma-induced coagulopathy by replenishing the lost coagulation factors, rather than diluting the casualty's remaining factors. The Israel Defense Force Medical Corps decided to use freeze-dried plasma (FDP) as the fluid of choice for casualties in hemorrhagic shock in the prehospital setting. The aim of our study is to compare the differences of coagulation, perfusion measurements, resource utilization, and outcome between casualties receiving FDP to casualties who did not receive FDP in the prehospital setting.
METHODS:
This is a retrospective matched cohort study based on two groups of casualties (those treated with FDP vs. those without FDP treatment). The control group was compiled in three steps of precision for age, sex, mechanism of injury and maximum level of severity for each nine injured body regions. Data were collected from the IDF Trauma Registry and The National Israel Trauma Registry.
RESULTS:
The study group comprised 48 casualties receiving FDP and 48 controls with no differences in demographic, evacuation time, and injury characteristics. The FDP group demonstrated a lower level of hemoglobin (12.7 gr/dzl) (odds ratio [OR], 3.11; 95% confidence interval [CI], 1.10-8.80), lower level of international normalized ratio (1.1) (OR, 3.09; 95% CI, 1.04-9.14), and lower level of platelets (230 × 109/L) (OR, 3.06; 95% CI, 1.16-8.06). No other differences were found between the two groups.
" In the total study population, seven (7.3%) casualties died in hospital, among which 8.3% were from the FDP group, compared with 6.2% from the control group"
CONCLUSION:
The use of FDP in the prehospital setting has logistic benefits and a positive effect on coagulation profile, with no other significant effects. Future studies need to be performed on larger groups to verify trends or nullify our hypotheses.