19/11/2019
PLyo à température ambiante: Pas si sûr !
Freeze-dried plasma stability under prehospital field conditions.
BACKGROUND:
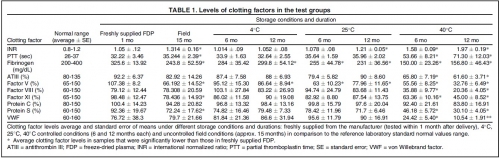
This study evaluated the effect of routine, uncontrolled, Israeli field storage conditions on the stability and efficacy of Lyo-Plas N freeze-dried plasma (FDP). We evaluated clotting factors V, VIII, and XI; proteins S and C; fibrinogen; partial thromboplastin time (PTT); antithrombin III (ATIII); von Willebrand factor (VWF); and international normalized ratio (INR) in FDP stored at 4°C, 25°C, and 40°C for 6 and 12 months, as well as FDP returned from field units after uncontrolled storage for 15 months (manufacturer's shelf life).
METHODS AND MATERIALS:
After reconstitution, clotting factor levels were compared to those of freshly supplied FDP doses.
RESULTS:
At 4°C for 12 months, factor V decreased slightly. At 25°C, average fibrinogen and factor V content were significantly lower at both periods, and INR was higher after 12 months. At 40°C, all samples were out of normal range in at least one clotting factor after 6 or 12 months. After field storage for 15 months, fibrinogen, factors V and XI, PTT, and protein S were significantly decreased, and INR increased.
However, these levels were still within laboratory norms. Statistically significant difference in clotting factors compared to laboratory normal range was found in INR (higher) and factor V (lower).
CONCLUSIONS:
Our data show minimal decreases in clotting factors in FDP after storage under field conditions, when compared to laboratory normal ranges. Along with the many advantages of FDP, this supports its use at the point of injury under battlefield conditions, despite uncontrolled storage environments. Under controlled storage conditions at 4°C, shelf life could possibly be extended, although further study is required.

Les commentaires sont fermés.