20/01/2022
Fluid Resuscitation in Tactical Combat Casualty Care. Update 201
Fluid Resuscitation in Tactical Combat Casualty Care
TCCC Guidelines Change 21-01
4 November 2021
Dans ce document le mot important est choc hémorragique. Ce n'est pas parce q'un blessé saigne qu'il est en état de choc.
| Tags : choc, hémorragie, transfusion
12/03/2020
Vasopresseurs: Pas si bon ?
Prehospital vasopressor use is associated with worse mortality in combat wounded.
------------------------------------------
Le recours à un vasopresseur est recommandé lors de la prise en charge du choc hémorragique. Le travail présenté qui porte sur le choc traumatique du blessé de guerre ne semble pas être en faveur de cette recommandation. Ceux recevant un vasopressueurs auraient une mortalité plus élevée. Ces résultats sont à prendre avec recul tant la fréquence de l'emploi de soutien hémodynamique apparait faible (0,4% des blessés) et de l'absence de données sur la temporalité de l'administration des vasopresseurs. De plus il semble exister une différence dans les mécanismes d'aggression (plus d'explosion dans le groupe avec soutien). Les auteurs évoquent l'intérêt de la vasopressine dont l'apport serait meilleur que celui de la noradrénaline et de l'adrénaline
------------------------------------------
Introduction:
Vasopressor medications are frequently used in the management of hypotension secondary to shock. However, little data exists regarding their use in hypotensive trauma patients and their use is controversial.
Methods:
The Department of Defense Trauma Registry was queried from January 2007 to August 2016 using a series of procedural codes to identify eligible casualties, which has been previously described. Mortality was compared between hypotensive casualties with documentation of receipt of vasopressor medications versus casualties not receiving vasopressors. To control for potential confounders, comparisons were repeated by constructing a multivariable logistic regression model including that utilized patient category, mechanism of injury, composite injury severity score, total blood products transfused, prehospital heart rate and prehospital systolic pressure. Survival was compared between these groups using propensity matching.
Results: Our search strategy yielded 28,222 patients, 124 (0.4%) of whom received prehospital vasopressors. On univariable analysis vasopressor use was associated with a lower odds of survival (OR 0.09, 0.06-0.13). The lower odds of survival persisted in the multivariate logistic regression model (OR 0.32, 0.18-0.56). Survival was lower among the vasopressor group (71.3%) when compared to a propensity matched cohort (94.3%).Conclusions: In this dataset, prehospital vasopressor use was associated with lower odds of survival. This finding persisted when adjusting for confounders and in a propensity matched cohort model.
| Tags : choc
09/10/2018
Voies aériennes et choc hémorragique, que faire ?
Airway and ventilation management strategies for hemorrhagic shock. To tube, or not to tube, that is the question!
Hudson AJ et Al. J Trauma Acute Care Surg. 2018 Jun;84(6S Suppl 1):S77-S82
------------------------------
Primum non nocere. Souvent ne pas faire parce que c'est le plus prudent ET NON PAS PARCE QU'ON N'A PAS APPRIS ET QU'ON NE SAIT DONC PAS FAIRE.
------------------------------
Many standard trauma management guidelines advocate the early use of endotracheal intubation (ETI) and positive pressure ventilation as key treatment interventions in hemorrhagic shock. The evidence for using these airway and ventilation strategies to manage a circulation problem is unclear. The potentially harmful effects of drug-assisted intubation and positive pressure ventilation include reduced cardiac output, apnea, hypoxia, hypocapnea (due to inadvertent hyperventilation), and unnecessarily prolonged on-scene times. Conversely, the beneficial effects of spontaneous negative pressure ventilation on cardiac output are well described. Few studies, however, have attempted to explore the potential advantages of a strategy of delayed intubation and ventilation (together with a policy of aggressive volume replacement) in shocked trauma patients. Given the lack of evidence, the decision making around how, when, and where to subject shocked trauma patients to intubation and positive pressure ventilation remains complex. If providers choose to delay intubation, they must have the appropriate skills to safely manage the airway and recognize the need for subsequent intervention. If they decide to perform intubation and positive pressure ventilation, they must understand the potential risks and how best to minimize them. We suggest that for patients with hemorrhagic shock who do not have a compromised airway and who are able to maintain adequate oxygen saturation (or mentation if monitoring is unreliable), a strategy of delayed intubation should be strongly encouraged.
| Tags : choc
08/05/2017
Hémorragie: De la glace sur le visage ?
Face Cooling Increases Blood Pressure during Simulated Blood Loss
B. Johnson et Al. Proceedings of Experimental Biology 2017 Chicago
-------------------------------------
Une constatation qui ne devrait pas surprendre ceux qui s'intéressent à la médecine de plongée et au réflexe d'immersion (1, 2, 3, 4), dont le facteur principal de déclenchement est l'exposition de la face à de l'eau froide.
-------------------------------------
Introduction
Blood loss causes central hypovolemia and in severe instances, it can decrease blood pressure and lead to cardiovascular decompensation. Simple and quick interventions that can be used to prevent cardiovascular decompensation in pre-hospital settings could be a valuable tool for first responsders. Cooling the forehead and cheeks using an ice/water slurry mixture has been shown to increase blood pressure for over 15 minutes. Therefore, face cooling could be used to mitigate decreases in blood pressure during blood loss.
Purpose
We tested the hypothesis that face cooling during simulated blood loss will increase blood pressure. Methods Ten healthy participants (22 ± 2 years, 3 women) completed two randomized trials on separate days. Both trials began with 30 mmHg of lower body negative pressure (LBNP) to simulate blood loss for 6 minutes. Then, either a 2.5 L plastic bag of an ice/water slurry mixture (0 ± 0°C) (LBNP+FC) or a 2.5 L plastic bag of thermoneutral water (34 ± 1°C) (LBNP+SHAM) was placed on the forehead and eyes and 30 mmHg of LBNP was maintained for an additional 15 minutes.

We continuously measured blood pressure (Penaz method), heart rate (ECG), stroke volume (Modelflow), cardiac output, total peripheral resistance, and forehead temperature throughout the protocol.
Results
Forehead temperature did not change from LBNP (34.2 ± 0.6°C) to LBNP+SHAM (33.9 ± 1.4°C, P > 0.999) and decreased from LBNP (34.4 ± 0.5°C) to LBNP+FC (11.0 ± 1.6°C, P < 0.001). Mean arterial pressure did not change from LBNP (82 ± 10 mmHg) to LBNP+SHAM (80 ± 8 mmHg, P = 0.978), but markedly increased during LBNP+FC. The peak increase from LBNP (77 ± 9 mmHg) was observed after 3 minutes of LBNP+FC (98 ± 15 mmHg, P < 0.001). Heart rate during LBNP (76 ± 14 bpm, P = 0.978) was not different from LBNP+SHAM (75 ± 13 bpm). Heart rate was lower throughout LBNP+FC beginning at 2 minutes of FC (60 ± 16 bpm) versus LBNP (80 ± 19 bpm, P < 0.001). Stroke volume did not change from LBNP (72 ± 15 mL) to LBNP+SHAM (67 ± 18 mL, P = 0.857). However, stroke volume increased from LBNP (78 ± 16 mL) to LBNP+FC, and peaked after 5 minutes of FC (97 ± 32 mL, P < 0.001). Cardiac output did not change from LBNP (5.4 ± 1.0 L/min) to LBNP+SHAM (4.9 ± 1.0 L/min, P > 0.415). Cardiac output slightly decreased from LBNP (6.2 ± 1.5 L/min) to 2 minutes of LBNP+FC (5.3 ± 1.6 L/min, P = 0.038). Total peripheral resistance did not change from LBNP (15.6 ± 3.7 mmHg/L/min) to LBNP+SHAM (17.3 ± 3.2 mmHg/L/min, P = 0.613). Total peripheral resistance throughout LBNP+FC was greater than LBNP. The peak increase in total peripheral resistance was observed after 2 minutes of LBNP+FC (20.0 ± 6.2 mmHg/L/min) versus LBNP (13.2 ± 3.9 mmHg/L/min, P < 0.001).
Conclusions
Face cooling during simulated moderate blood loss increases blood pressure through an increase in total peripheral resistance. Although more research is warranted, face cooling during blood loss is a potential simple and quick intervention that could delay cardiovascular decompensation. Support or Funding InformationUniversity at Buffalo IMPACT Award
| Tags : choc, hémorragie
23/03/2017
REBOA: Une technique qui trouve sa place
Resuscitative endovascular balloon occlusion of the aorta for uncontrolled haemorrhagic shock as an adjunct to haemostatic procedures in the acute care setting
-------------------------------
Le principe d'occlusion artérielle n'est pas nouveau. L'occlusion endovasculaire de l'aorte trouve sa place dans la gestion des hémorragies abdominales incontrôlable. Son intérêt en traumatologie ballistique de guerre est probable (1). Cette technique a été récemment décrite en phase préhospitalière (2). Nos conditions d'exercice méritent que l'on s'intéresse à cette technique (3).
-------------------------------
Background:
Haemorrhagic shock is a major cause of death in the acute care setting. Since 2009, our emergency department has used intra-aortic balloon occlusion (IABO) catheters for resuscitative endovascular balloon occlusion of the aorta (REBOA).
Methods: REBOA procedures were performed by one or two trained acute care physicians in the emergency room (ER) and intensive care unit (ICU). IABO catheters were positioned using ultrasonography. Collected data included clinical characteristics, haemorrhagic severity, blood cultures, metabolic values, blood transfusions, REBOA-related complications and mortality.
Results: Subjects comprised 25 patients (trauma, n = 16; non-trauma, n = 9) with a median age of 69 years and a median shock index of 1.4. REBOA was achieved in 22 patients, but failed in three elderly trauma patients. Systolic blood pressure significantly increased after REBOA (107 vs. 71 mmHg, p < 0.01). Five trauma patients (20 %) died in ER, and mortality rates within 24 h and 60 days were 20 % and 12 %, respectively. No REBOA-related complications were encountered. The total occlusion time of REBOA was significantly lesser in survivors than that in non-survivors (52 vs. 97 min, p < 0.01). Significantly positive correlations were found between total occlusion time of REBOA and shock index (Spearman’s r = 0.6) and lactate concentration (Spearman’s r = 0.7) in survivors.
Conclusion: REBOA can be performed in ER and ICU with a high degree of technical success. Furthermore, correlations between occlusion time and initial high lactate levels and shock index may be important because prolonged occlusion is associated with a poorer outcome.
| Tags : choc, hémorragie
10/12/2016
Maintenir la pression: Hydroxocobalamine ou adré ?
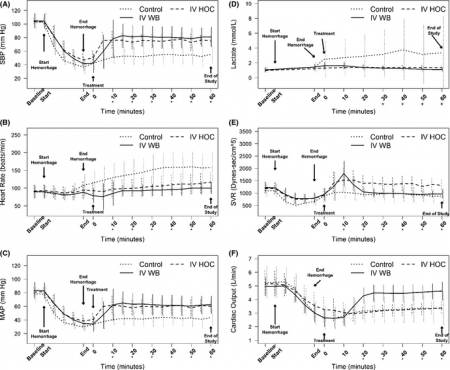
A prospective, randomized trial of intravenous hydroxocobalamin versus whole blood transfusion compared to no treatment for Class III hemorrhagic shock resuscitation in a prehospital swine model.
OBJECTIVES:
The objective was to compare systolic blood pressure (sBP) over time in swine that have had 30% of their blood volume removed (Class III shock) and treated with intravenous (IV) whole blood or IV hydroxocobalamin, compared to nontreated controlanimals.
METHODS:
Thirty swine (45 to 55 kg) were anesthetized, intubated, and instrumented with continuous femoral and pulmonary artery pressure monitoring. Animals were hemorrhaged a total of 20 mL/kg over a 20-minute period. Five minutes after hemorrhage, animals were randomly assigned to receive 150 mg/kg IV hydroxocobalamin solubilized in 180 mL of saline, 500 mL of whole blood, or no treatment. Animals were monitored for 60 minutes thereafter. A sample size of 10 animals per group was determined based on a power of 80% and an alpha of 0.05 to detect an effect size of at least a 0.25 difference (>1 standard deviation) in mean sBP between groups. sBP values were analyzed using repeated-measures analysis of variance (RANOVA). Secondary outcome data were analyzed using repeated-measures multivariate analysis of variance (RMANOVA).
RESULTS:
There were no significant differences between hemodynamic parameters of IV hydroxocobalamin versus whole blood versus control group at baseline (MANOVA; Wilks' lambda; p = 0.868) or immediately posthemorrhage (mean sBP = 47 mm Hg vs. 41 mm Hg vs. 37 mm Hg; mean arterial pressure = 39 mm Hg vs. 28 mm Hg vs. 34 mm Hg; mean serum lactate = 1.2 mmol/L vs. 1.4 mmol/L vs. 1.4 mmol/L; MANOVA; Wilks' lambda; p = 0.348). The outcome RANOVA model detected a significant difference by time between groups (p < 0.001). Specifically, 10 minutes after treatment, treated animals showed a significant increase in mean sBP compared to nontreated animals (mean sBP = 76.3 mm Hg vs. 85.7 mm Hg vs. 51.1 mm Hg; p < 0.001). RMANOVA modeling of the secondary data detected a significant difference in mean arterial pressure, heart rate, and serum lactate (p < 0.001). Similar to sBP, 10 minutes after treatment, treated animals showed a significant increase in mean arterial pressure compared to nontreated animals (mean arterial pressure = 67.7 mm Hg vs. 61.4 mm Hg vs. 40.5 mm Hg). By 10 minutes, mean heart rate was significantly slower in treated animals compared to nontreated animals (mean heart rate = 97.3 beats/min vs. 95.2 beats/min vs. 129.5 beats/min; p < 0.05). Serum lactate, an early predictor of shock, continued to rise in the control group, whereas it did not in treated animals. Thirty minutes after treatment, serum lactate values of treated animals were significantly lower compared to nontreated animals (p < 0.05). This trend continued throughout the 60-minute observation period such that 60-minute values for lactate were 1.4 mmol/L versus 1.1 mmol/L versus 3.8 mmol/L. IV hydroxocobalamin produced a statistically significant increase in systemic vascular resistance compared to control, but not whole blood, with a concomitant decrease in cardiac output.
CONCLUSIONS:
Intravenous hydroxocobalamin was more effective than no treatment and as effective as whole blood transfusion, in reversing hypotension and inhibiting rises in serum lactate in this prehospital, controlled, Class III swine hemorrhage model.
| Tags : choc
17/01/2016
Cyanokit pour l'hémorragie massive ?
A prospective, randomized trial of intravenous hydroxocobalamin versus whole blood transfusion compared to no treatment for class III hemorrhagic shock resuscitation in a prehospital swine model
Bebarta VS et Al. Acad Emerg Med.2015 Mar;22(3):321-30
--------------------------------
La prise en charge des hémorragies traumatiques en préhospitalier est basée sur la miseen oeuvre des moyens d'arrêts de ces dernières et l'initiation d'une stratégie raisonnée de remplissage vasculaire et de transfusion. L'apport équilibrée de CGR, de plasma,de fractions coagulantes et même de plaquettes fait partie de cette démarche de damage control resuscitation de même que l'apport d'acide tranexaminique pour s'opposer à une fibrinolyse précoce souvent présente. D'autres axes de recherches sont proposés. AInsi l'hydroxocobalamine, connue en tant qu'antidote de l'acide cynahydrique permettrait sur des cochons auxquels on aurait soustrait 20 ml/kg de sang de préserver la pression artérielle et la lactatémie de manière identique à celle obtenue par l'apport de sang total.
--------------------------------
OBJECTIVES:
The objective was to compare systolic blood pressure (sBP) over time in swine that have had 30% of their blood volume removed (Class III shock) and treated with intravenous (IV) whole blood or IV hydroxocobalamin, compared to nontreated control animals.
METHODS:
Thirty swine (45 to 55 kg) were anesthetized, intubated, and instrumented with continuous femoral and pulmonary artery pressure monitoring. Animals were hemorrhaged a total of 20 mL/kg over a 20-minute period. Five minutes after hemorrhage, animals were randomly assigned to receive 150 mg/kg IV hydroxocobalamin solubilized in 180 mL of saline, 500 mL of whole blood, or no treatment. Animals were monitored for 60 minutes thereafter. A sample size of 10 animals per group was determined based on a power of 80% and an alpha of 0.05 to detect an effect size of at least a 0.25 difference (>1 standard deviation) in mean sBP between groups. sBP values were analyzed using repeated-measures analysis of variance (RANOVA). Secondary outcome data were analyzed using repeated-measures multivariate analysis of variance (RMANOVA).
RESULTS:
There were no significant differences between hemodynamic parameters of IV hydroxocobalamin versus whole blood versus control group at baseline (MANOVA; Wilks' lambda; p = 0.868) or immediately posthemorrhage (mean sBP = 47 mm Hg vs. 41 mm Hg vs. 37 mm Hg; mean arterial pressure = 39 mm Hg vs. 28 mm Hg vs. 34 mm Hg; mean serum lactate = 1.2 mmol/L vs. 1.4 mmol/L vs. 1.4 mmol/L; MANOVA; Wilks' lambda; p = 0.348). The outcome RANOVA model detected a significant difference by time between groups (p < 0.001). Specifically, 10 minutes after treatment, treated animals showed a significant increase in mean sBP compared to nontreated animals (mean sBP = 76.3 mm Hg vs. 85.7 mm Hg vs. 51.1 mm Hg; p < 0.001). RMANOVA modeling of the secondary data detected a significant difference in mean arterial pressure, heart rate, and serum lactate (p < 0.001). Similar to sBP, 10 minutes after treatment, treated animals showed a significant increase in mean arterial pressure compared to nontreated animals (mean arterial pressure = 67.7 mm Hg vs. 61.4 mm Hg vs. 40.5 mm Hg). By 10 minutes, mean heart rate was significantly slower in treated animals compared to nontreated animals (mean heart rate = 97.3 beats/min vs. 95.2 beats/min vs. 129.5 beats/min; p < 0.05). Serum lactate, an early predictor of shock, continued to rise in the control group, whereas it did not in treated animals. Thirty minutes after treatment, serum lactate values of treated animals were significantly lower compared to nontreated animals (p < 0.05). This trend continued throughout the 60-minute observation period such that 60-minute values for lactate were 1.4 mmol/L versus 1.1 mmol/L versus 3.8 mmol/L. IV hydroxocobalamin produced a statistically significant increase in systemic vascular resistance compared to control, but not whole blood, with a concomitant decrease in cardiac output.
CONCLUSIONS:
Intravenous hydroxocobalamin was more effective than no treatment and as effective as whole blood transfusion, in reversing hypotension and inhibiting rises in serum lactate in this prehospital, controlled, Class III swine hemorrhage model.
| Tags : choc
19/11/2015
Stratégie Low Flow: Encore confirmée
Efficacy of limited fluid resuscitation in patients with hemorrhagic shock: a meta-analysis
Duan C. et Al. Int J Clin Exp Med. 2015; 8(7): 11645–11656.

| Tags : remplissage, choc, hémorragie
23/10/2015
Quelle place pour les facteurs de la coagulation ?
| Tags : hémorragie, coagulopathie, choc
07/02/2015
RFE Choc hémorragique
| Tags : choc, hémorragie
04/09/2014
Remplissage vasculaire: Evolution majeure du TCCC
L'emploi préhospitalier de la transfusion de globules rouges et de plasma était évoqué de manière anecdotique. Une évolution importante survient dans la procédure américaine du TCCC (1, 2). Cette pratique est en passe de devenir une recommandation protocolée de théâtre pour les blessés en état de choc (Pas de pouls radial et conscience altérée el l'absence de traumatisme crânien) hémorragique avec notons le recours au Plyo du CTSA.
" Tactical Field Care and TACEVAC Care
7. Fluid resuscitation
a. The resuscitation fluids of choice for casualties in hemorrhagic shock, listed from most to least preferred, are: whole blood*; plasma, RBCs and platelets in 1:1:1 ratio*; plasma and RBCs in 1:1 ratio; plasma or RBCs alone; Hextend; and crystalloid (Lactated Ringers or Plasma-Lyte A).
b. Assess for hemorrhagic shock (altered mental status in the absence of brain injury and/or weak or absent radial pulse).
1. If not in shock:
- No IV fluids are immediately necessary.
- Fluids by mouth are permissible if the casualty is conscious and can swallow.
2. If in shock and blood products are available under an approved command or theater blood product administration protocol:
- Resuscitate with whole blood*, or, if not available
- Plasma, RBCs and platelets in a 1:1:1 ratio*, or, if not available
- Plasma and RBCs in 1:1 ratio, or, if not available;
- Reconstituted dried plasma, liquid plasma or thawed plasma alone or RBCs alone;
- Reassess the casualty after each unit. Continue resuscitation until a palpable radial pulse, improved mental status or systolic BP of 80-90 mmHg is present.
3. If in shock and blood products are not available under an approved command or theater blood product administration protocol due to tactical or logistical constraints:
- Resuscitate with Hextend, or if not available;
- Lactated Ringers or Plasma-Lyte A;
- Reassess the casualty after each 500 mL IV bolus;
- Continue resuscitation until a palpable radial pulse, improved mental status, or systolic BP of 80-90 mmHg is present.
- Discontinue fluid administration when one or more of the
above end points has been achieved.
4. If a casualty with an altered mental status due to suspected TBI has a weak or absent peripheral pulse, resuscitate as necessary to restore and maintain a normal radial pulse. If BP monitoring is available, maintain a target systolic BP of at least 90 mmHg.
5. Reassess the casualty frequently to check for recurrence of shock. If shock recurs, recheck all external hemorrhage control measures to ensure that they are still effective and repeat the fluid resuscitation as outlined above.
* Neither whole blood nor apheresis platelets as these products are currently collected in theater are FDA-compliant. Consequently, whole blood and 1:1:1 resuscitation using apheresis platelets should be used only if all of the FDA-compliant blood products needed to support 1:1:1 resuscitation are not avalaible
| Tags : choc, coagulopathie, remplissage
02/12/2012
Expansion volémique par F Shortgen IAR IDF
| Tags : remplissage, choc
Choc hémorragique par D. Journois IAR IDF
| Tags : choc, hémorragie, remplissage
08/09/2012
Moins remplir: Mieux ? Oui, MAIS
Restrictive fluid resuscitation in combination with damage control resuscitation: Time for adaptation.
Marquinn D et all. J Trauma Acute Care Surg. 2012;73: 674-678.
-------------------------------------------------------------------------------------------------------------
Ce travail confirme l'intérêt d'une stratégie de remplissage vasculaire parcimonieuse dès lors qu'une réanimation et une chirurgie spécialisée moderne sont accessibles. Le collectif des patients est ici celui de traumatisés thoraciques sévères arrivant dans une structure spécialisée dans des délais très courts. De telles conditions ne sont pas forcément celles de la prise en charge de blessés de guerre pour qui les délais d'évacuation sont souvent plus élevés. Il n'en demeure pas moins que cette publication milite pour une politique de remplissage vasculaire raisonnée. On rappelle que la procédure du sauvetage au combat donne pour objectif la perception d'un pouls radial perceptible.
-------------------------------------------------------------------------------------------------------------
BACKGROUND:
Damage control resuscitation (DCR) conveys a survival advantage in patients with severe hemorrhage. The role of restrictive fluid resuscitation (RFR) when used in combination with DCR has not been elucidated. We hypothesize that RFR, when used with DCR, conveys an overall survival benefit for patients with severe hemorrhage.
METHODS:
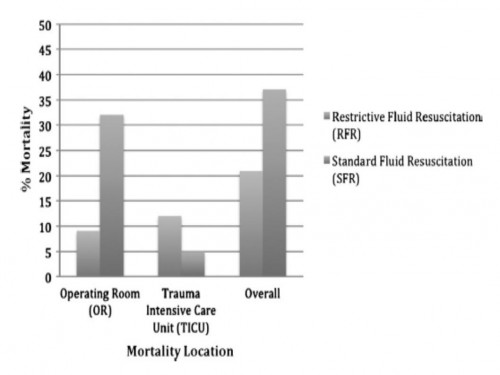
This is a retrospective analysis from January 2007 to May 2011 at a Level I trauma center. Inclusion criteria included penetrating torso injuries, systolic blood pressure less than or equal to 90 mm Hg, and managed with DCR and damage control surgery (DCS). There were two groups according to the quantity of fluid before DCS: (1) standard fluid resuscitation (SFR)greater than or equal to 150 mL of crystalloid; (2) RFR less than 150 mL of crystalloid. Demographics and outcomes were analyzed.
RESULTS:
Three hundred seven patients were included. Before DCS, 132 (43%) received less than 150 mL of crystalloids, grouped under RFR; and 175 (57%) received greater than or equal to 150 mL of crystalloids, grouped under SFR. Demographics and initial clinical characteristicswere similar between the study groups. Compared with the SFR group, RFR patients received less fluid preoperatively (129 mL vs. 2,757 mL; p G 0.001), exhibited a lower intraoperative mortality (9% vs. 32%; p G 0.001), and had a shorter hospital length of stay (13 vs. 18 days; p = 0.02). Patients in the SFR group had a lower trauma intensive care unit mortality (5 vs. 12%; p = 0.03) but exhibited a higher overall mortality. Patients receiving RFR demonstrated a survival benefit, with an odds ratio for mortality of 0.69 (95% confidence interval, 0.37Y0.91).
CONCLUSION:
To the best of our knowledge, this is the first civilian study that analyzes the impact of RFR in patients managed with DCR. Its use in conjunction with DCR for hypotensive trauma patients with penetrating injuries to the torso conveys an overall and early intraoperative survival benefit.
| Tags : remplissage, choc, hémorragie, perfusion
24/12/2011
HEA, vraiment sûrs ?: Réponse début 2012 ?
Fluid Resuscitation with 6% Hydroxyethyl Starch (130/0.4) in Acutely Ill Patients: An Updated Systematic Review and Meta-Analysis
Gattas DJ et all. Anesth Analg 2012; 114:159–69
Une autre méta-analyse sur l'inocuité ou pas des solutions d'HEA 130 000/0,4 (Voluven]. Rien ne permet de conclure pour ou pas. Les auteurs estiment nécessaire de s'assurer de l'inocuité réele de ces produits par des travaux conduits de manière indépendante de l'industrie et sur une population plus large que celle de réanimation habituellement incluse. Ils mettent par ailleurs en avant le retrait récent de plusieurs publications portant sur la sécurité de ce type d'HEA. Il cite une étude intitulée Crystalloid versus Hydroxyethyl Starch Trial (CHEST) réalisée en australie portant sur l'analyse de la mortalité à 90 jours en comparant ce type de solutés à du salé isotonique qui devrait voir ses premiers résultats produits début 2012.
| Tags : remplissage, perfusion, choc
08/12/2011
Document SFMU: Choc hémorragique en médecine de l'avant
| Tags : choc, hémorragie, remplissage, garrot, tourniquet
21/12/2010
Hydroxyethylamidon: Attention !
Cette revue de littérature rappelle quelques données essentielles sur l'emploi des HEA pour le remplissage vasculaire. Il en ressort que le bien fondé de cette pratique n'est pas validé y compris pour les HEA de 3ème génération 130/0,4 dont fait partie le voluven pourtant les moins sujets à complications. Ces dernières sont repréesntées par le risque d'insuffisance rénale, la tendance au saignement et une tendance à l'augmentation de la mortalité. Il n'est pas sans intérêt de faire remarquer que l'HYPERHES est un soluté contennat un HEA de type 2005.
Première lecture
REVIEW ARTICLE
The Efficacy and Safety of Colloid Resuscitation in the Critically Ill
Christiane S. Hartog, MD, Michael Bauer, MD, and Konrad Reinhart, MD
(Anesth Analg 2011;112:156 –64)
Despite evidence from clinical studies and meta-analyses that resuscitation with colloids or crystalloids is equally effective in critically ill patients, and despite reports from high-quality clinical trials and meta-analyses regarding nephrotoxic effects, increased risk of bleeding, and a trend toward higher mortality in these patients after the use of hydroxyethyl starch (HES) solutions, colloids remain popular and the use of HES solutions is increasing worldwide. We investigated the major rationales for colloid use, namely that colloids are more effective plasma expanders than crystalloids, that synthetic colloids are as safe as albumin, that HES solutions have the best risk/benefit profile among the synthetic colloids, and that the third-generation HES 130/0.4 has fewer adverse effects than older starches. Evidence from clinical studies shows that comparable resuscitation is achieved with considerably less crystalloid volumes than frequently suggested, namely, 2-fold the volume of colloids. Albumin is safe in intensive care unit patients except in patients with closed head injury. All synthetic colloids, namely, dextran, gelatin, and HES have dose-related side effects, which are coagulopathy, renal failure, and tissue storage. In patients with severe sepsis, higher doses of HES may be associated with excess mortality. The assumption that third-generation HES 130/0.4 has fewer adverse effects is yet unproven. Clinical trials on HES 130/0.4 have notable shortcomings. Mostly, they were not performed in intensive care unit or emergency department patients, had short observation periods of 24 to 48 hours, used cumulative doses below 1 daily dose limit (50 mL/kg), and used unsuitable control fluids such as other HES solutions or gelatins. In conclusion, the preferred use of colloidal solutions for resuscitation of patients with acute hypovolemia is based on rationales that are not supported by clinical evidence. Synthetic colloids are not superior in critically ill adults and children but must be considered harmful depending on the cumulative dose administered. Safe threshold doses need to be determine in studies in high-risk patients and observation periods of 90 days. Such studies on HES 130/0.4 are still lacking despite its widespread and increasing use. Because there are safer and
equally effective alternatives in the form of crystalloids, use of synthetic colloids should be avoided except in the context of clinical studies.
Seconde lecture
La Société Française d'anesthésie-réanimation rappelle d'ailleurs également ce risque hémorragique y compris pour les HEA 10/0,4 notamment lors d'adminsitration aiguë.
"
L’albumine, les gélatines et l’amidon 130 000/0,4 (Voluven®) n’interfèrent pas, ou quasiment pas avec l’hémostase. La prudence reste toutefois conseillée : Comentaires Ainsi les effets secondaires des HEA ne sont pas que liés à eur adminsitration répétitive. Dans nos conditions d'emploi , il apparait nécessaire d'observer une certaine retenue dans ll'usage des HEA. Ceci plaide pour l'utilisation des solutés cristalloïdes tel que le Ringer lactate, le sérum salé hypertonique et pose la question d'un recours plus large au gélatines fluides modifiées malgré leur risque allergique (?)
- chez les patients insuffisants rénaux
- en cas de perfusion prolongée ou de volume de colloïdes important ;
- en cas de troubles de l’hémostase acquis ;
- chez les patients traités par antiplaquettaires ou anticoagulants ;
- en présence d’une anémie ou thrombopénie profonde ;
- chez les patients porteurs d’une maladie de Willebrand ;
- chez les patients de groupe sanguin O (ces patients ont spontanément un taux de Willebrand plus bas) ;
- en présence d’un saignement chirurgical actif ;
- en présence d’une transfusion massive ou d’une hypothermie.
| Tags : remplissage, choc, hémorragie
17/10/2009
La voie humérale pour la perfusion intraosseuse
| Tags : choc, intraosseux
Le BIG: Emploi
| Tags : intraosseux, choc
12/08/2009
BIG et son maintien
L'emploi du BIG est intéressant. Cependant il faut savoir que dans la "vrai vie", sa pose est effective dans moins de 70% des cas. Il est particulièrement important de maintenir très fermement le dispositif au moment de l'impact. En effet le ressort qui propulse l'aiguille est comprimé à environ 20kg. Il existe donc un "recul" potentiellement important lors de la mise en oeuvre. Il faut donc appuyer très fortement de façon à ce que l'impact se fasse bien perpendiculairement à l'os.
| Tags : intraosseux, choc