07/11/2022
Remplissage vasculaire: Quid dans la vrai vie US ?
Trends in Prehospital Blood, Crystalloid, and Colloid Administration in Accordance With Changes in Tactical Combat Casualty Care Guidelines
Clarke E E. et Al. Military Medicine, Volume 187, Issue 11-12, November-December 2022, Pages e1265–e1270,
Introduction
Hemorrhage is the leading threat to the survival of battlefield casualties. This study aims to investigate the types of fluids and blood products administered in prehospital trauma encounters to discover the effectiveness of Tactical Combat Casualty Care (TCCC) recommendations.
Materials and Methods
This is a secondary analysis of a previously described dataset from the Department of Defense Trauma Registry with a focus on prehospital fluid and blood administration in conjunction with changes in the TCCC guidelines. We collected demographic information on each patient. We categorized receipt of each fluid type and blood product as a binary variable for each casualty and evaluated trends over 2007–2020 both unadjusted and controlling for injury severity and mechanism of injury.
Results
Our original dataset comprised 25,897 adult casualties from January 1, 2007 through March 17, 2020. Most (97.3%) of the casualties were male with a median age of 25. Most (95.5%) survived to hospital discharge, and 12.2% of the dataset received fluids of any kind. Medical personnel used crystalloids in 7.4% of encounters, packed red blood cells in 2.0%, and whole blood in 0.5% with very few receiving platelets or freeze-dried plasma. In the adjusted model, we noted significant year-to-year increases in intravenous fluid administration from 2014 to 2015 and 2018 to 2019, with significant decreases noted in 2008–2009, 2010–2012, and 2015–2016. We noted no significant increases in Hextend used, but we did note significant decreases in 2010–2012. For any blood product, we noted significant increases from 2016 to 2017, with decreases noted in 2009–2013, 2015–2016, and 2017–2018. Overall, we noted a general spike in all uses in 2011–2012 that rapidly dropped off 2012–2013. Crystalloids consistently outpaced the use of blood products. We noted a small upward trend in all blood products from 2017 to 2019.
Conclusions
Changes in TCCC guidelines did not immediately translate into changes in prehospital fluid administration practices. Crystalloid fluids continue to dominate as the most commonly administered fluid even after the 2014 TCCC guidelines changed to use of blood products over crystalloids. There should be future studies to investigate the reasons for delay in guideline implementation and efforts to improve adherence.
| Tags : remplissage, transfusion
04/03/2022
Transfusion de sang frais
Use of Walking Blood Bank at Point of Injury During Combat Operations. A Case Report
Gaddy M et Al. J Spec Oper Med. Winter 2021;21(4):94-98
The US Military Tactical Combat Casualty Care guidelines recommend blood products as the preferred means of fluid resuscitation in trauma patients;, however, most combat units do not receive blood products prior to executing combat operations. This is largely due to logistical limitations in both blood supply and transfusion equipment. Further, the vast majority of medics are not trained in transfusion protocol. For many medics, the logistical constraints for cold-stored blood products favor the use of Walking Blood Bank (WBB), however few cases have been reported of WBB implementation at the point of injury during real world combat operations. This case report reviews one case of successful transfusion using WBB procedures at point of injury during combat. It highlights not only the feasibility, but also the necessity, for implementation of this practice on a larger scale.
| Tags : transfusion
20/01/2022
Fluid Resuscitation in Tactical Combat Casualty Care. Update 201
Fluid Resuscitation in Tactical Combat Casualty Care
TCCC Guidelines Change 21-01
4 November 2021
Dans ce document le mot important est choc hémorragique. Ce n'est pas parce q'un blessé saigne qu'il est en état de choc.
| Tags : choc, hémorragie, transfusion
23/12/2017
Le glycoalyx: Une cible thérapeutique
-----------------------------------
La correction précoce de la coagulopathie traumatique est un des objectifs fondamentaux de la prise en charge du traumatisé sévère et en particulier des blessés de guerre. L'emploi de plasma lyophilisé / fibrinogène / fractions coagulantes concentrées et la transfusion de sang frais se fait selon des stratégies diverses visant à compenser les déficits observés (1) . La restauration d'une perméabilité capillaire est également une voie possible. On peut considérer que l'efficacité du plasma est en +/- grande partie liée à sa capacité à restaurer le glycocalyx endothélial (2). Le travail présenté est en faveur de l'emploi, à l'instar de l'acide tranexaminique pour la fibrinolyse, d'ajuvants pharmacologiques aux solutés de remplissage pour limiter l'atteinte du glycoclayx.
Allez sur le forum coagulopathie/transfusion
Mieux comprende le concept de perméabilité capillaire
-----------------------------------
INTRODUCTION:
There is interest in the small-volume therapeutic use of adjunct drugs for treating hemorrhagic shock (HS). However, critical information is only partially available on mechanisms of action of promising compounds such as adenosine-lidocaine-magnesium (ALM), beta-hydroxybutyrate plus melatonin (BHB/M), and poloxamer 188 (P-188). Therefore, we tested the hypothesis that these adjuncts would reverse HS-induced damage to microvascular endothelial glycocalyx and hemodynamics.
METHODS:
After baseline, 40% of total blood volume was removed from 44 anesthetized Sprague-Dawley male rats. One hour after hemorrhage, animals were resuscitated using ALM, BHB/M, or P-188 followed by lactated Ringer's (LR, 15 mL/kg). Control animals were not treated (SHAM) or received LR alone. Sampled blood was used to quantify shed syndecan-1 in plasma; multiple systemic physiological parameters were recorded. In vivo glycocalyx thickness, microvascular permeability, and microhemodynamics were evaluated in >200 cremaster venules using intravital videomicroscopy.
RESULTS:
Compared with baseline, resuscitation using adjuncts was associated with glycocalyx restoration of 97 ± 9% (ALM), 75 ± 8% (BHB/M), and 85 ± 5% (P-188): significantly higher than LR-only (56 ± 4%). Significantly better permeability, similar to SHAM values, was measured after ALM and P-188, and low plasma syndecan-1 levels were measured after resuscitation with all adjuncts. Microhemodynamic changes were relatively small while systemic parameters such as mean arterial pressure and lactate improved but remained below or above the baseline, respectively, as expected from this hypotensive resuscitation model.
CONCLUSION:
The drugs ALM, BHB/M, and P-188 provide beneficial effects as adjuncts to hypotensive resuscitation in this HS model by mechanisms involving changes at the microvascular level including the glycocalyx.
| Tags : coagulopathie, transfusion
01/12/2017
Transfusion à l'avant: Surtout Plyo, et le sang ?
Early transfusion on battlefield before admission to role 2: A preliminary observational study during “Barkhane” operation in Sahel
Vitalys V. et Al. https://doi.org/10.1016/j.injury.2017.11.029
---------------------------------------
Ce document rapporte essentiellement l'emploi de PLyo chez des blessés de guerre avant leur prise en charge en role 2. Beaucoup est écrit sur la faisabilité d'une telle pratique. On rappelle que l'emploi du plasma lyophilisé n'est pas une nouveauté y compris en milieu précaire (1). Le plasma lyophilisé était d'emploi très courant dans les années 70 et début 80, à un tel point qu'il était pratiquement utilisé comme soluté de remplissage chez le traumatisé routier grave. Il s'agit donc d'une redécouverte et on n'est pas surpris que ceci puisse se retrouver 30 ans après avec un produit notoirement sécurisé (2).
Il est dommage que l'article ne détaille pas plus cet emploi au niveau des role 1 qui est le lieu de début de transfusion pour 5 des blessés transfusés sur 7, ce qui n'est pas vraiment mis en avant dans ce travail. La reconstitution du Plyo est simple, mais probablement moins aisée dans une voilure tournante que dans un Casanurse ou au sol. Cet article met également en avant l'absence de transfusion de sang total, ce qui est étonnant pour une pratique rentrée dans les moeurs chez nos collègues US et UK. Enfin on retrouve l'emploi de fibrinogène, dont l'apport suffisant ne peut être assuré par le PLyo seul, et que certains proposent en première ligne de fractions coagulantes (3). Chose intéressante deux échecs de reconstitution du Plyo ont été observées.
Un article très intéressant, dont plusieurs auteurs sont en charge du développement du PLyo, avec dans la partie discussion un bon rappel des problématiques qui se posent dans de telles circonstances. Il est dommage que le regroupement des lésions du bassin avec l'atteinte des membres ait été fait car les problématiques de contrôle des hémorragies sont différentes. De même la description des lésions observées dans le groupe transfusé (qui ne sont pas tous porteurs d'un garrot), et l'analyse spécifique du groupe présentant une hémorragie encore active aurait été méritées.
---------------------------------------
Introduction
Haemorrage is the leading cause of death after combat related injuries and bleeding management is the cornerstone of management of these casualties. French armed forces are deployed in Barkhane operatio n in the Sahel-Saharan Strip who represents an immense area. Since this constraint implies evacuation times beyond doctrinal timelines, an institutional decision has been made to deploy blood products on the battlefield and transfuse casualties before role 2 admission if indicated. The purpose of this study was to evaluate the transfusion practices on battlefield during the first year following the implementation of this policy.
Materials and methods
prospective collection of data about combat related casualties categorized alpha evacuated to a role 2. Battlefield transfusion was defined as any transfusion of blood product (red blood cells, plasma, whole blood) performed by role 1 or Medevac team before admission at a role 2. Patients’ characteristics, battlefield transfusions’ characteristics and complications were analysed.
Results
During the one year study, a total of 29 alpha casualties were included during the period study. Twenty-eight could be analysed, 7/28 (25%) being transfused on battlefield, representing a total of 22 transfusion episodes. The most frequently blood product transfused was French lyophilized plasma (FLYP). Most of transfusion episodes occurred during medevac. Compared to non-battlefield transfused casualties, battlefield transfused casualties suffered more wounded anatomical regions (median number of 3 versus 2, p = 0.04), had a higher injury severity score (median ISS of 45 versus 25, p = 0,01) and were more often transfused at role 2, received more plasma units and whole blood units. There was no difference in evacuation time to role 2 between patients transfused on battlefield and non-transfused patients. There was no complication related to battlefield transfusions. Blood products transfusion onset on battlefield ranged from 75 min to 192 min after injury.
Conclusion
Battlefield transfusion for combat-related casualties is a logistical challenge. Our study showed that such a program is feasible even in an extended area as Sahel-Saharan Strip operation theatre and reduces time to first blood product transfusion for alpha casualties. FLYP is the first line blood product on the battlefield
| Tags : transfusion
31/08/2017
TXA: Peut être pas si évident
Military use of TXA in combat trauma: Does it matter?
BACKGROUND:
Tranexamic acid (TXA) has been previously reported to have a mortality benefit in civilian and combat-related trauma, and was thus added to the Joint Theater Trauma System Damage Control Resuscitation Clinical Practice Guideline. As part of ongoing system-wide performance improvement, the use of TXA has been closely monitored. The goal was to evaluate the efficacy and safety of TXA use in military casualties and provide additional guidance for continued use.
METHODS:
A total of 3,773 casualties were included in this retrospective, observational study of data gathered from a trauma registry. The total sample, along with 3 sub-samples for massive transfusion patients (n=784), propensity-matched sample (n=1,030) and US/NATO military (n=1,262), were assessed for administration of TXA and time from injury to administration of TXA. Outcomes included mortality and occurrence of pulmonary embolism (PE) and deep vein thrombosis (DVT). Multivariable proportional hazards regression models with robust standard error estimates were used to estimate hazard ratios (HR) for assessment of outcomes while controlling for covariates.
RESULTS:
Results of univariate and multivariate analyses of the total sample (HR=0.97; 95%CI 0.62-1.53; p=0.86); massive transfusion sample (HR=0.84; 95%CI 0.46-1.56; p=0.51); propensity-matched sample (HR=0.68; 95%CI 0.27-1.73; p=0.34); and US/NATO military sample (HR=0.76; 95%CI 0.30-1.92; p=0.48) indicate no statistically significant association between TXA use and mortality. Use of TXA was associated with increased risk of PE in the total sample (HR=2.82; 95%CI 2.08-3.81; p<0.001); massive transfusion sample (HR=3.64; 95%CI 1.96-6.78; p=0.003); US/NATO military sample (HR=2.55; 95%CI 1.73-3.69; p=0.002); but not the propensity-matched sample (HR=3.36; 95%CI 0.80-14.10; p=0.10). TXA was also associated with increased risk of DVT in the total sample (HR=2.00; 95%CI 1.21-3.30; p=0.02) and US/NATO military sample (HR=2.18; 95%CI 1.20-3.96; p=0.02).
CONCLUSIONS:
In the largest study on TXA use in a combat trauma population, TXA was not significantly associated with mortality, due to lack of statistical power. However, our HR estimates for mortality among patients who received TXA are consistent with previous findings from the CRASH2 trial. At the same time, continued scrutiny and surveillance of TXA use in military trauma, specifically for prevention of thromboembolic events, is warranted.
| Tags : transfusion
22/01/2017
Du sang qui descend du ciel
Nous ne sommes pas les seuls à avoir à faire face au contexte d'isolement. Il est particulièrement intéressant de regarder comment ces problèmes sont abordés par les pays en voie de construction. L'exemple rwandais devrait nous interpeller. Le recours à des drones de livraison en contexte militaire n'est pas une utopie car ce mode de ravitaillement a été utilisé en afghanistan.
| Tags : transfusion
21/01/2017
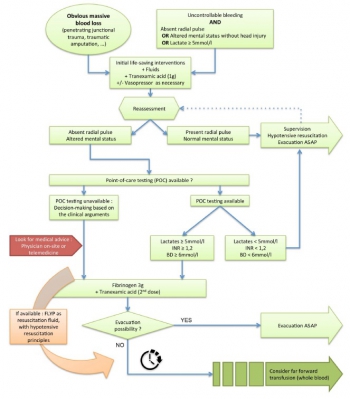
Coagulopathie: Fibrinogène avant PLyo, mais les 2 et + tôt ?
Despite the early uses of tourniquets and haemostatic dressings, blood loss still accounts for the vast majority of preventable deaths on the battlefield. Over the last few years, progress has been made in the management of such injuries, especially with the use of damage control resuscitation concepts. The early application of these procedures, on the field, may constitute the best opportunity to improve survival from combat injury during remote operations.
DATA SOURCES:
Currently available literature relating to trauma-induced coagulopathy treatment and far-forward transfusion was identified by searches of electronic databases. The level of evidence and methodology of the research were reviewed for each article. The appropriateness for field utilisation of each medication was then discussed to take into account the characteristics of remote military operations.
CONCLUSIONS:
In tactical situations, in association with haemostatic procedures (tourniquet, suture, etc), tranexamic acid should be the first medication used according to the current guidelines. The use of fibrinogen concentrate should also be considered for patients in haemorrhagic shock, especially if point-of-care (POC) testing of haemostasis or shock severity is available. If POC evaluation is not available, it seems reasonable to still administer this treatment after clinical assessment, particularly if the evacuation is delayed. In this situation, lyophilised plasma may also be given as a resuscitation fluid while respecting permissive hypotension. Whole blood transfusion in the field deserves special attention.
In addition to the aforementioned treatments, if the field care is prolonged, whole blood transfusion must be considered if it does not delay the evacuation
| Tags : coagulopathie, transfusion
15/12/2016
Sang total: Pas que chaud, de banque aussi
Coagulation function of stored whole blood is preserved for 14 days in austere conditions: A ROTEM feasibility study during a Norwegian antipiracy mission and comparison to equal ratio reconstituted blood.
BACKGROUND:
Formulation of a medical preparedness plan for treating severely bleeding casualties during naval deployment is a significant challenge because of territory covered during most missions. The aim of this study was to evaluate the concept of "walking blood bank" as a supportable plan for supplying safe blood and blood products.
METHODS:
In 2013, the Royal Norwegian Navy conducted antipiracy operations from a frigate, beginning in the Gulf of Aden and ending in the Indian Ocean. Crews were on 24-hour emergency alert in preparation for an enemy assault on the frigate. Under an approved command protocol, a "walking blood bank," using crew blood donations, was established for use on board and on missions conducted in rigid-hulled inflatable boats, during which freeze-dried plasma and leukoreduced, group O low anti-A/anti-B titer, cold-stored whole blood were stored in Golden Hour Boxes. Data demonstrating the ability to collect, store, and provide whole blood were collected to establish feasibility of implementing a whole blood-focused remote damage-control resuscitation program aboard a naval vessel. In addition, ROTEM data were collected to demonstrate feasibility of performing this analysis on a large naval vessel and to also measure hemostatic efficacy of cold-stored leukoreduced whole blood (CWB) stored during a period of 14 days. ROTEM data on CWB was compared with reconstituted whole blood.
RESULTS:
Drills simulating massive transfusion activation were conducted, in which 2 U of warm fresh whole blood with platelet sparing leukoreduction were produced in 40 minutes, followed by collection of two additional units at 15-minute increments. The ROTEM machine performed well during ship-rolling, as shown by the overlapping calculated and measured mechanical piston movements measured by the ROTEM device. Error messages were recorded in 4 (1.5%) of 267 tests. CWB yielded reproducible ROTEM results demonstrating preserved fibrinogen function and platelet function for at least 3.5 weeks and 2 weeks, respectively. The frequency of ROTEM tests were as follows: EXTEM (n = 88), INTEM (n = 85), FIBTEM (n = 82), and APTEM (n = 12). CWB results were grouped. Compared with Days 0 to 2, EXTEM maximum clot firmness was significantly reduced, beginning on Days 10 to 14; however, results through that date remained within reference ranges and were comparable with the EXTEM maximum clot firmness for the reconstituted whole blood samples containing Day 5 room temperature-stored platelets.
CONCLUSION:
A "walking blood bank" can provide a balanced transfusion product to support damage-control resuscitation/remote damage-control resuscitation aboard a frigate in the absence of conventional blood bank products. ROTEM analysis is feasible to monitor damage-control resuscitation and blood product quality. ROTEM analysis was possible in challenging operational conditions.
| Tags : coagulopathie, transfusion
24/09/2016
Transfusion en vol: Sécurité assurée
Risk Management Analysis of Air Ambulance Blood Product Administration in Combat Operations
BACKGROUND:
Between June-October 2012, 61 flight-medic-directed transfusions took place aboard U.S. Army Medical Evacuation (medevac) helicopters in Afghanistan. This represents the initial experience for pre-hospital blood product transfusion by U.S. Army flight medics.
METHODS:
We performed a retrospective review of clinical records, operating guidelines, after-action reviews, decision and information briefs, bimonthly medical conferences, and medevac-related medical records.
RESULTS:
A successful program was administered at 10 locations across Afghanistan. Adherence to protocol transfusion indications was 97%. There were 61 casualties who were transfused without any known instance of adverse reaction or local blood product wastage. Shock index (heart rate/systolic blood pressure) improved significantly en route, with a median shock index of 1.6 (IQR 1.2-2.0) pre-transfusion and 1.1 (IQR 1.0-1.5) post-transfusion (P < 0.0001). Blood resupply, training, and clinical procedures were standardized across each of the 10 areas of medevacoperations.
DISCUSSION:
Potential risks of medical complications, reverse propaganda, adherence to protocol, and diversion and/or wastage of limited resources were important considerations in the development of the pilot program. Aviation-specific risk mitigation strategies were important to ensure mission success in terms of wastage prevention, standardized operations at multiple locations, and prevention of adverse clinical outcomes. Consideration of aviation risk mitigation strategies may help enable other helicopter emergency medical systems to develop remote pre-hospital transfusion capability. This pilot program provides preliminary evidence that blood product administration by medevac is safe.
| Tags : transfusion
04/06/2016
PLyo: Une révolution ? Pas vraiment, une redécouverte
Pusateri AE et Al. Transfusion. 2016 Apr;56 Suppl 2:S128-39
------------------------------------------
Les nouvelles modalités de transfusion mettent en avant le bénéfice de l'apport précoce de plasma. Les contraintes logistiques liées à l'emploi de plasma frais sont réelles. L'emploi de plasma lyophilisé permet de raccourcir ce délai et peut représenter dans certaines conditions d'isolement la seule source disponibles de fractions coagulantes. Le plasma lyophylisé est un vieux monsieur, mais dont la place est fondamentale. Largement utilisé notamment par l'armée française pendant la guerre d'indocchine, le SSA a maintenu sa production jusqu'à ce que l'épidémie de VIH ne survienne. Depuis les années 1980, le SSA a travaillé sans relâche pour sécuriser un produit qui retrouve la place qui lui est due dans la stratégie transfusionnelle du blessé de guerre (1) Il s'agit donc d'une redécouverte avec un emploi effectif en opération dès 1996 (2), plutôt que de révolution. Le document proposé à la lecture fait le point sur cette historique et les développements à venir. La lecture de ce document ne doit pas faire oublier la réflexion de plus en plus présente sur l'emploi en situation d'isolement de l'intérêt de la transfusion de sang total, seule source de plaquettes, associé au recours à des fractions coagulantes comme le fibrinogène et les complexes prothrombiques. Une telle association représente probablement l'avenir de la réanimation hémostatique préhospitalière (3, 4).
------------------------------------------
Historical dried plasma development Event Selected References
1930s Plasma lyophilization developed in the 1930s.
1940—Large scale production of pooled, lyophilized plasma by both the US and British established for war time use (to meet logistical constraints of whole blood and frozen/liquid plasma).ans les années
1941—Spray dried plasma produced for the Swedish Defense Department. 21 WWII Production 20-22 British produced >500,000 U lyophilized plasma during WWII. US produced >6,000,000 U lyophilized plasma during WWII. US/British distributed world-wide. Sweden produced approximately 17,000 U spray dried plasma for Sweden and Finland.
1945—Hepatitis 23 Hepatitis as a result of plasma transfusion recognized by the end of WWII. Believed that benefits outweighed the risk.
1945-1952—Hepatitis 24 Attempts at pathogen reduction and reducing pool size not successful. Several deaths in clinical studies of ultraviolet irradiated pooled plasma.
1953
—Department of the Army (Circular 73) directed that, because of the risk of serum hepatitis, the higher cost, and the need to use it for the production of specific globulins, plasma would not be used “to support blood volume” unless dextran was not available.
—Serum albumin replaced plasma as primary resuscitative product for US Forces in Korea.
1968—National Research Council Committee on Plasma and Plasma Substitutes recommended that “the use of whole, pooled human plasma be discouraged and even discontinued unless a clear cut case can be made for its unique requirements.”
The French Military Blood Institute produced dried plasma from 1949 to 1984, and provided over 40,000 units to French military forces during the Indochina War. In 1985, production was discontinued due to risk of HIV infection.
| Tags : coagulopathie, transfusion
03/06/2016
The 2015 Remote Damage Control Resuscitation Symposium
| Tags : transfusion, coagulopathie
11/04/2016
PROPPR Study: 1-1-2 aussi bien !
Damage-control resuscitation and emergency laparotomy: Findings from the PROPPR study
Undurraga VJ et AL. J Trauma Acute Care Surg. 2016 Apr;80(4):568-75
BACKGROUND:
The Pragmatic Randomized Optimal Platelet and Plasma Ratios (PROPPR) trial has demonstrated that damage-controlresuscitation, a massive transfusion strategy targeting a balanced delivery of plasma-platelet-red blood cell in a ratio of 1:1:1, results in improved survival at 3 hours and a reduction in deaths caused by exsanguination in the first 24 hours compared with a 1:1:2 ratio. In light of these findings, we hypothesized that patients receiving 1:1:1 ratio would have improved survival after emergency laparotomy.
METHODS:
Severely injured patients predicted to receive a massive transfusion admitted to 12 Level I North American trauma centers were randomized to 1:1:1 versus 1:1:2 as described in the PROPPR trial. From these patients, the subset that underwent an emergency laparotomy, defined previously in the literature as laparotomy within 90 minutes of arrival, were identified. We compared rates and timing of emergency laparotomyas well as postsurgical survival at 24 hours and 30 days.
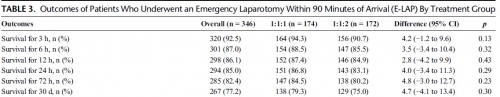
RESULTS:
Of the 680 enrolled patients, 613 underwent a surgical procedure, 397 underwent a laparotomy, and 346 underwent an emergency laparotomy. The percentages of patients undergoing emergency laparotomy were 51.5% (174 of 338) and 50.3% (172 of 342) for 1:1:1 and 1:1:2, respectively (p = 0.20). Median time to laparotomy was 28 minutes in both treatment groups. Among patients undergoing an emergency laparotomy, the proportions of patients surviving to 24 hours and 30 days were similar between treatment arms; 24-hour survival was 86.8% (151 of 174) for 1:1:1 and 83.1% (143 of 172) for 1:1:2 (p = 0.29), and 30-day survival was 79.3% (138 of 174) for 1:1:1 and 75.0% (129 of 172) for 1:1:2 (p = 0.30).
CONCLUSION:
We found no evidence that resuscitation strategy affects whether a patient requires an emergency laparotomy, time to laparotomy, or subsequent survival.
| Tags : coagulopathie, transfusion