31/10/2014
HES: Du côté des belges
Hydroxyethyl Starches in the Perioperative Period. A review on the efficacy and safety of starch solutions
Ghuselings I et All. Acta Anaesth. Belg., 2014, 65, 9-22

Garrot: Pas de jeu avant de serrer
No slackers in tourniquet use to stop bleeding
Polston RW et Al. J Spec Oper Med. 2013 Summer;13(2):12-9.
La performance d'un garrot tient pour beaucoup à la manière dont il va être posé. Le garrot en dotation comporte un dispositif de serrage de type tourniquet. L'efficacité de ce dispositif est en grande partie liée à l'absence de jeu au niveau de la sangle du garrot avant le serrage. Il est fondamental de positionner le garrot à la racine du membre, de le mettre en tension manuellement en tirant sur la sangle de façon à effectuer un premier serrage du garrot et ensuite de renforcer ce serrage par la barre du tourniquet. On rappelle qu'en principe l'efficacité est obtenue à partir de 3 tours. (la fiche technique). Le travail présenté ci après est éloquent sur l'intérêt de prohiber tout jeu avec de tourner la barre de torsion.
----------------------------------------------------------------------------------
Background: Tourniquets on casualties in war have been loose in 4%?9% of uses, and such slack risks death from uncontrolled bleeding. A tourniquet evidence gap persists if there is a mechanical slack?performance association.
Objective: The purpose of the present study was to determine the results of tourniquet use with slack in the strap versus no slack before windlass turning, in order to develop best practices. Methods: The authors used a tourniquet manikin 254 times to measure tourniquet effectiveness, windlass turns, time to stop bleeding, and blood volume lost at 5 degrees of strap slack (0mm, 25mm, 50mm, 100mm, and 200mm maximum).
Results: When comparing no slack (0mm) to slack (any positive amount), there were increases with slack in windlass turns (p < .0001, 3-fold), time to stop bleeding (p < .0001, 2-fold), and blood volume lost (p < .0001, 2-fold). When comparing no slack to 200mm slack, the median results showed an increase in slack for windlass turns (p < .0001), time to stop bleeding (p < .0001), and blood volume lost (p < .0001).
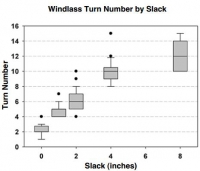
Conclusions: Any slack presence in the strap impaired tourniquet performance. More slack had worse results. Trainers can now instruct tourniquet users with concrete guidance.
| Tags : garrot, tourniquet
Garrot: Comment positionner la boucle ?
Emergency tourniquet effectiveness in four positions on the proximal thigh.
Kragh JF et Al. J Spec Oper Med. 2014 Spring;14(1):26-9.
La procédure du sauvetage au combat précise que le garrot doit être posé à la racine du membre. Elle ne précise cependant pas comment le dispositif de serrage doit être placé: en avant sur le côté en arrière du membre. Ce travail tente de répondre à cette question.
----------------------------------------------------------------------------------
Objective: The purpose of the present study is to determine the performance of tourniquet use by the placement of the tourniquet's windlass on the extremity in four positions: medial, lateral, anterior, and posterior? to inform tourniquet instructors and develop best tourniquet practices.
Methods: A HapMed™ Leg Tourniquet Trainer was used as a manikin to test the effectiveness of an emergency tourniquet, the Special Operations Forces Tactical Tourniquet. Two users made 10 tests, each in four positions.
Results: Effectiveness rates of tourniquet use were 100% in all four positions. The two tourniquet users were both right-hand dominant and used their right hand to turn the windlass. One user turned the windlass clockwise, and the other turned it counterclockwise. The association between time to stop bleeding and tourniquet position was statistically significant but associations between time to stop bleeding and the user, user-by-position, and windlass turn number were not statistically significant. The association between tourniquet position and pressure under the tourniquet was statistically significant, and the association between user and pressure under thetourniquet was statistically significant, but the user-by-position and windlass turn number were not statistically significant. The associations betweentourniquet position and blood loss volume, user and blood loss volume, and user-by-position and blood loss volume were statistically significant. Conclusions: The present study found that tourniquet effectiveness rates were uniformly 100% irrespective of whether the windlass position was medial, lateral, anterior, or posterior. These excellent clinical and statistical results indicate that users may continue to place the tourniquets as they prefer upon the proximal thigh
| Tags : tourniquet, garrot
Garrot jonctionnel: CROC et SAM ?
Testing of junctional tourniquets by military medics to control simulated groin hemorrhage.
Kragh JF et Al. J Spec Oper Med. 2014 Fall;14(3):58-63.
Ce qui est certain, c'est ce qui est écrit sur cette image

Difficile d'avoir une idée précise sur les performances relatives des matériels proposés par les industriels. Ce qui suit éclaire un peu.
BACKGROUND:
Junctional hemorrhage is a common cause of death on the battlefield, but there is no documented direct comparison for the use of junctional tourniquet models by US medics. The purpose of this testing is to assess military medic experience with the use of junctional tourniquets in simulated out-of-hospital trauma care.
METHODS:
Nine medics (seven men and two women) used four different junctional tourniquets: Combat Ready Clamp™ (CRoC™; http://www.combatmedicalsystems.com), Abdominal Aortic and Junctional Tourniquet™ (AAJT™; http://www.compressionworks.net), Junctional Emergency Treatment Tool (JETT™; http://www.narescue .com), and SAM Junctional Tourniquet® (SJT®; http:// www.sammedical.com/products). These medics also acted as simulated casualties. Effectiveness percentages, as measured by stopped distal pulse by Doppler auscultation, and time to effectiveness were recorded in two tests per tourniquet (72 total tests). Tourniquet users ranked their preference of model by answering the question: "If you had to go to war today and you could only choose one, which tourniquet would you choose to bring?"
RESULTS:
All tourniquets used were safe under the conditions of this study. Both the SJT and the CRoC had high effectiveness percentages; their rate difference was not statistically significant. The SJT and the CRoC had fast times to effectiveness; their time difference was not statistically significant. Users preferred the SJT and the CRoC; their ranked difference was not statistically significant.
CONCLUSION:
The SJT and the CRoC were equally effective and fast and were preferred by the participants
| Tags : jonctionnel
30/10/2014
Plaquettes synthétiques: Possible ? Mais oui
Tuning Ligand Density on Intravenous Hemostatic Nanoparticles Dramatically Increases Survival Following Blunt Trauma
Proc Natl Acad Sci U S A. 2014 Jul 15;111 (28) 10293-10298
Des plaquettes synthétiques pour arrêter le saignement ? Certains l'ont fait grâce à la technologie des nanoparticules (1, 2). Un espoir qui reste à confirmer.
-----------------------------------------------------------------------------------
Explosions account for 79% of combat-related injuries, leading to multiorgan hemorrhage and uncontrolled bleeding. Uncontrolled bleeding is the leading cause of death in battlefield traumas as well as in civilian life. We need to stop the bleeding quickly to save lives, but, shockingly, there are no treatments to stop internal bleeding. A therapy that halts bleeding in a site-specific manner and is safe, stable at room temperature, and easily administered is critical for the advancement of trauma care. To address this need, we have developed hemostatic nanoparticles that are administered intravenously. When tested in a model of blast trauma with multiorgan hemorrhaging, i.v. administration of the hemostatic nanoparticles led to a significant improvement in survival over the short term (1 h postblast). No complications from this treatment were apparent out to 3 wk. This work demonstrates that these particles have the potential to save lives and fundamentally change trauma care.
-----------------------------------------------------------------------------------
| Tags : hémorragie
The Blue protocol
Lung ultrasound in the critically ill
Lichtenstein D. Annals of Intensive Care 2014, 4:1
Une approche systématisée de l'échographie pulmonaire simpllfie et fiabilise cet examen. C'est ce qu'explique cette publication
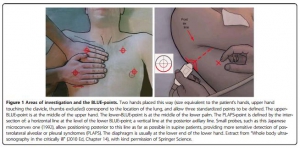
27/10/2014
Perfuser + vite: Pas n'importe comment !
Novel rapid infusion device for patients in emergency situations
Kapoor DJ et Al. Scand J Trauma Resusc Emerg Med. 2011 Jun 10;19:35
Il peut être nécessaire d'accélérer le débit d'une perfusion. Pour cela il peut être fait appel à une manchette à pression, une tubulure de type 'blood pump", un système "robinet 3 voies ", plus rarement un système à compression par lame voire une pompe électrique.
Le document présenté fait appel à l'injection d'air dans le corps du soluté utilisé. La sécurité de cette manière de faire interpelle quelque peu. En effet les auteurs estiment que le filtre 15µ présent dans la tubulure a un effet barrière anti air suffisant pour éviter tout risque d'embolie gazeuse.
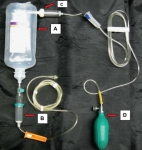 Ce type de pratique est associé à une prise de risque du fait du concept même de la méthode, des conditions d'hygiène non respectées et des conditions de surveillance non optimales en conditions extrême.
Ce type de pratique est associé à une prise de risque du fait du concept même de la méthode, des conditions d'hygiène non respectées et des conditions de surveillance non optimales en conditions extrême.
23/10/2014
Pansements hémostatiques: Actualisation 2014
Review of New Topical Hemostatic Dressings for Combat Casualty Care
Benett BL et Al Mil Med. 2014 May;179(5):497-514
This review analyzes the new (2008-2013) hemostatic agents and dressings for enhanced efficacy in preclinical studies, and investigates supportive findings among case reports of effectiveness and safety in hospital and prehospital literature. A literature search was conducted using PubMed, National Library of Medicine using key words and phrases. The search revealed a total of 16 articles that fit the criteria established for third-generation hemostatic dressings. There were a total of 9 preclinical, 5 clinical, and 2 prehospital studies evaluated. Evaluation of these third- generation studies reveals that mucoadhesive (chitosan) dressings, particularly Celox Gauze and ChitoGauze, clearly show equal efficacy to Combat Gauze across many dependent variables. Chitosan-based products are ideal prehospital dressings because they are shown to work independently from the physiological clotting mechanisms. Many first-, second-, and third-generation chitosan-based dressings have been in use for years by the United States and other NATO militaries at the point of injury, and during tactical evacuation, in Operation Enduring Freedom and Operation Iraqi Freedom without reported complications or side effects. Based on the reported efficacy and long-term safety of chitosan-based products, increased use of Celox Gauze and ChitoGauze within the Department of Defense and civilian venues merits further consideration and open debate.
| Tags : hémorragie, pansement
14/10/2014
Pansements hémostatiques: Actualisation 2014 TCCC
Management of External Hemorrhage in Tactical Combat Casualty Care:
Chitosan-Based Hemostatic Gauze Dressings
TCCC Guidelines – Change 13-05
Le point sur l'emploi des pansements hémostatiques. Pas de grands changements le Quikclot Combat Gauze reste la référence mais le Chitosan et le Celox gauze sont considérés comme équivalents.
Cliquer sur le lien pour accéder à l'argumentaire
TCCC%20Bennett%20Hemostatic%20Drsngs%20TCCC%20Change%20JS...
11/10/2014
Suis je bien dans la trachée ?
Comment vérifier la position intratrachéale d'une sonde d'intubation ?
Le débat n'est pas nouveau (1). L'intérêt de l'intubation en condition de combat est d'ouvrir les voies aériennes, de prévenir le risque d'inhalation et de permettre l'application d'une ventilation adéquate. Son indication doit être bien mesurée car elle va ajouter une dimension de complexité pour un transport préhospitalier qui n'a rien à voir avec ce qui est rencontré en métropole.
Alors quelques réflexions ne sont pas inutiles car il faut éviter tant l'intubation oesophagienne que l'intubation sélective.
1. La visualisation de la sonde entre les cordes vocales est la base que ce soit au moment de la laryngoscopie initiale ou d'un contrôle après MAIS ce n'est pas suffisant, et pas forcément toujours possible.
2. La recherche d'une auscultation symétrique des 2 champs pulmonaires et d'un silence auscultatoire épigastrique doivent être fait MAIS ce n'est pas suffisant, et parfois difficile à obtenir.
3. L'expansion thoracique symétrique et la constatation de buée sur la sonde sont observées MAIS ce n'est pas suffisant.
4. Le recours à la mesure d'une SaO2 MAIS n'est pas du tout fait pour cela.
5. Utiliser une seringue ou un bulbe spécifique MAIS n'offre pas de certitude et c'est une techniQue peu diffusée en France
6. La CERTITUDE de l'intubation est donnée par la constatation de CO2 dans le gaz expiré à condition que soient respectés des critères quantitatifs et qualitatifs. Notamment les capnogrammes doivent être visualisés sur au moins 6 cycles ventilatoires durant lesquels l’absence de décroissance du signal confirme la bonne position de la sonde. (accéder à la conférence de consensus de la SFAR). Cette analyse qualitative est importante car le CO2 observé peut provenir d'air gastrique insufflé lors de ventilation manuelle voire d'anti-acides gastriques.
Mais dans notre contexte d'emploi la capnographie, telle que l'on la connait au bloc opératoire ou en SAUV, n'est pas le plus souvent disponible. Vous disposez de capteurs chimiques qui ne donnent qu'une estimation de la capnométrie. Certains disposent d'un capnomètre portable mais qui ne donne pas d'informations qualitatives. Enfin il existe des détecteurs oesophagiens dont la performance est bonne (2), sous réserve de conditions de stockage et d'emploi conforme notamment de température ambiante, mais qui ne permettent pas une surveillance continue et surtout si ils sont fiables pour confirmer la position intra-trachéale, ils le sont beaucoup moins pour la position intra-oeosophagienne.
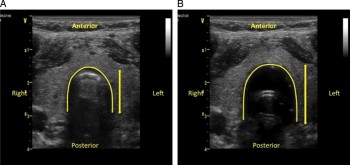
7. Du nouveau arrive avec l'emploi de l'échographie pour valider la position intra-trachéale de la sonde d'intubation:
- soit par échographie cervicale (3, 4),
Kerforne T et al. Br. J. Anaesth. 2013;111:510-511
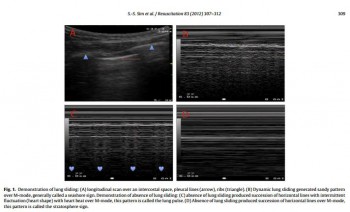
- soit par constatation de mouvements pleuraux bilatéraux (5)
- ou encore d'une mobilité diaphragmatique lors de la ventilation (6)
Int J Crit Illn Inj Sci. 2013 Apr;3(2):113-7
En contexte Militaire et en l'absence de capnographe, il faut, avant fixation de la sonde de vérification, dans le cadre la réalisation de mesures primaires de vérification de la bonne position de la sonde d'intubation ASSOCIER l'observation directe de la sonde franchissant les cordes vocales, l'expansion symétrique du thorax lors de la ventilation au ballon, la présence de buée dans la sonde, l'auscultation symétrique des deux champs pulmonaires et la présence de CO2 expiré sur le détecteur chimique. L'apport de l'échographie pourrait être d'être l'alternative à la radiographie pulmonaire pour la vérification secondaire de la bonne position de la sonde d'intubation.
Un débat qui porte sur la meilleure performance comparée à la capnographie et l'auscultation du repérage échographique est même déjà ouvert (7, 8, 9).
| Tags : airway
06/10/2014
Laryngoscopie directe: Toujours la référence en 1ère intention
Use of the Airtraq laryngoscope for emergency intubation in the prehospital setting: a randomized control trial.
Trimmel H et all. Crit Care Med 2011 Mar;39(3):489-93
__________________________________________
Une étude un peu ancienne mais qui a depuis été confirmée par d'autres (1, 2) et qui met bien en avant l'importance d'un apprentissage renforcé de la gestion des voies aériennes. Une méta-analyse plus récente le confirme (3).
__________________________________________
OBJECTIVES
The optical Airtraq laryngoscope (Prodol Meditec, Vizcaya, Spain) has been shown to have advantages when compared with direct laryngoscopy in difficult airway patients. Furthermore, it has been suggested that it is easy to use and handle even for inexperienced advanced life support providers. As such, we sought to assess whether the Airtraq may be a reliable alternative to conventional intubation when used in the prehospital setting.
DESIGN, SETTING, AND PATIENTS:
Prospective, randomized control trial in emergency patients requiring endotracheal intubation provided by anesthesiologists or emergency physicians responding with an emergency medical service helicopter or ground unit associated with the Department of Anesthesiology, General Hospital, Wiener Neustadt, Austria.
MEASUREMENTS AND MAIN RESULTS:
During the 18-month study period, 212 patients were enrolled. When the Airtraq was used as first-line airway device (n=106) vs. direct laryngoscopy (n=106), success rate was 47% vs. 99%, respectively (p<.001). Reasons for failed Airtraq intubation were related to the fiber-optic characteristic of this device (i.e., impaired sight due to blood and vomitus, n=11) or to assumed handling problems (i.e., cuff damage, tube misplacement, or inappropriate visualization of the glottis, n=24). In 54 of 56 patients where Airtraq intubation failed, direct laryngoscopy was successful on the first attempt; in the remaining two and in one additional case of failed direct laryngoscopy, the airway was finally secured employing the Fastrach laryngeal mask. There was no correlation between success rates and body mass index, age, indication for airway management, emergency medical service unit, or experience of the physicians.
CONCLUSIONS:
Based on these results, the use of the Airtraq laryngoscope as a primary airway device cannot be recommended in the prehospital setting without significant clinical experience obtained in the operation room. We conclude that the clinical learning process of the Airtraq laryngoscope is much longer than reported in the anesthesia literature.
| Tags : airway