03/06/2025
Drone et transfusion à l'avant
Massive transfusion on the combat field using autonomous drones: A case report
Türkoğlu B et Al . Transfusion. 2025 May 10. doi: 10.1111/trf.18279.
----------------------------------
Le transport de dérivés sanguins par drone est effectif et évalué depuis plusieurs années (1) notamment au Rwanda (2). Le cas rapporté est celui d'un blessé par IED. Le transport vers un role 1 est certainement d'un grand intérêt (4), cependant l'utilisation à grande échelle lors de conflit à haute intensité est certainement un sujet d'intérêt mais très probablement avec des objectifs plus larges et différents (5, 6)
Pour aller plus loin sur l'emploi des drones en médecine préhospitalière (7)
----------------------------------
Background:
Hemorrhage is a leading cause of preventable deaths in combat settings, requiring rapid blood transfusion to improve survival. While the feasibility of drone-assisted medical logistics has been explored, its practical application in battlefield transfusion remains unreported.
Study design and methods:
This case report describes the first documented massive transfusion in a combat environment using an autonomous unmanned aerial vehicle (UAV). A 27-year-old soldier sustained severe lower limb injuries from an improvised explosive device explosion and developed hemorrhagic shock in a remote battlefield location where adverse weather conditions prevented immediate evacuation. In response, 6 units of whole blood and 2 units of fresh frozen plasma were transported via UAV, enabling prehospital transfusion under telemedicine supervision.
Results:
The UAV successfully delivered blood products on two consecutive flights, ensuring early resuscitation and stabilization despite delayed evacuation. The casualty's vital signs improved post-transfusion, and surgical interventions were successfully performed following hospital admission. This case demonstrates the feasibility of drone-assisted blood product transport in prolonged field care scenarios.
Discussion:
To our knowledge, this is the first reported case of a combat casualty receiving a UAV-facilitated massive transfusion in an operational setting. While UAV-based medical logistics offers a rapid and reliable alternative for remote trauma care, challenges remain in regulatory implementation, blood product stability, and integration into standardized protocols. Further controlled studies are needed to optimize UAV-assisted transfusion strategies and their potential expansion into civilian and disaster response settings.
| Tags : drone
12/11/2024
PLyo: Oui mais sans hémodilution par les crstalloïdes
Prehospital Lyophilized Plasma Transfusion for Trauma-Induced Coagulopathy in Patients at Risk for Hemorrhagic Shock. A Randomized Clinical Trial
Jost D. et Al.JAMA Netw Open. 2022;5(7):e2223619.
-----------------
Le recours au plasma fait partie de l'arsenal thérapeutique de la prise en charge des traumatisés sévères en choc hémorragique. Comme l'étude REPHILL (1), Ce travail ne met pas en évidence d'amélioration de la survie à 28 jours. Les délais de transport et le degré d'hémodilution lié au recours aux solutés salés expliquent peut être cela (2)
Key Points
Question Does prehospital transfusion of lyophilized plasma result in a lower incidence of trauma-induced coagulopathy at hospital admission compared with standard care with normal saline infusion in patients at risk for hemorrhagic shock after trauma?
Findings This multicenter randomized clinical trial included 150 patients with trauma who were treated in a prehospital setting. Median international normalized ratio at hospital admission, massive transfusion rate, 30-day survival, and adverse events did not significantly differ between lyophilized plasma transfusion and standard care.
Meaning These findings suggest that prehospital lyophilized plasma transfusion is a feasible and safe procedure for patients who are at risk for hemorrhagic shock, although there is a lack of evidence regarding its ability to prevent trauma-induced coagulopathy.
Abstract
Importance Blood transfusion is a mainstay of therapy for trauma-induced coagulopathy, but the optimal modalities for plasma transfusion in the prehospital setting remain to be defined.
Objective To determine whether lyophilized plasma transfusion can reduce the incidence of trauma-induced coagulopathy compared with standard care consisting of normal saline infusion.
Design, Setting, and Participants This randomized clinical trial was performed at multiple centers in France involving prehospital medical teams. Participants included 150 adults with trauma who were at risk for hemorrhagic shock and associated coagulopathy between April 1, 2016, and September 30, 2019, with a 28-day follow-up. Data were analyzed from November 1, 2019, to July 1, 2020.
Intervention Patients were randomized in a 1:1 ratio to receive either plasma or standard care with normal saline infusion (control).
Main Outcomes and Measures The primary outcome was the international normalized ratio (INR) on arrival at the hospital. Secondary outcomes included the need for massive transfusion and 30-day survival. As a safety outcome, prespecified adverse events included thrombosis, transfusion-related acute lung injury, and transfusion-associated circulatory overload.
Results Among 150 randomized patients, 134 were included in the analysis (median age, 34 [IQR, 26-49] years; 110 men [82.1%]), with 68 in the plasma group and 66 in the control group. Median INR values were 1.21 (IQR, 1.12-1.49) in the plasma group and 1.20 (IQR, 1.10-1.39) in the control group (median difference, −0.01 [IQR, −0.09 to 0.08]; P = .88). The groups did not differ significantly in the need for massive transfusion (7 [10.3%] vs 4 [6.1%]; relative risk, 1.78 [95% CI, 0.42-8.68]; P = .37) or 30-day survival (hazard ratio for death, 1.07 [95% CI, 0.44-2.61]; P = .89). In the full intention-to-treat population (n = 150), the groups did not differ in the rates of any of the prespecified adverse events.
Conclusions and Relevance In this randomized clinical trial including severely injured patients at risk for hemorrhagic shock and associated coagulopathy, prehospital transfusion of lyophilized plasma was not associated with significant differences in INR values vs standard care with normal saline infusion. Nevertheless, these findings show that lyophilized plasma transfusion is a feasible and safe procedure for this patient population.
24/09/2024
Plasma; Et l'OctaplasLG ?
Une alternative HOSPITALIERE au PLyo du fait d'une production actuelle limitée ? VIent d'obtenir son agréement en Europe. Plus simple en gestion car considéré comme un médicament et non un produit sanguin. Il est fourni en présentations distinctes, selon les groupes sanguins. L’administration d’OCTAPLASLG doit respecter les règles de compatibilité des groupes sanguins ABO. En cas d’urgence, OCTAPLASLG du groupe AB peut être considéré comme plasma universel dans la mesure où il peut être administré à tous les patients, indépendamment de leur groupe sanguin. C'est donc dans cette forme qu'il peut être utilisé en préhospitalier comme le Plyo.
Sa place reste à préciser
10/06/2024
Fibrinogène précoce: Moins de transfusion ?
Hemostatic effect of fibrinogen concentrate on traumatic massive hemorrhage: a propensity score matching study
Heo HJ et al. Trauma Surg Acute Care Open. 2024 Jan 30;9(1):e001271.
Background:
Fibrinogen concentrate (FC) can be administered during massive transfusions to manage trauma-induced coagulopathy. However, its effectiveness in survival remains inconclusive due to scarce high-level evidence. This study aimed to investigate the hemostatic effects of FC regarding mortality in massive hemorrhage caused by trauma.
Methods:
This retrospective study analyzed 839 patients who received massive transfusions (red blood cells (RBCs) ≥5 units in 4 hours or ≥10 units in 24 hours) at a level I trauma center between 2015 and 2022. Patients who were transferred to other hospitals or were deceased upon arrival, suffered or died from severe brain injury, and were aged 15 years or less were excluded (n=334). 1:2 propensity score matching was performed to compare the 'FC (+)' group who had received FC in 24 hours (n=68) with those who had not ('FC (-)', n=437). The primary outcome was mortality, and the secondary outcomes included transfusion volume.
Results:
The variables for matching included vital signs, injury characteristics, prehospital time, implementation of resuscitative endovascular balloon occlusion of the aorta, and blood gas analysis results. The administration of FC did not significantly reduce or predict mortality (in-hospital, 24 hours, 48 hours, or 7 days). The FC (-) group received more units of RBC (25.69 units vs. 16.71 units, p<0.001, standardized mean difference [SMD] 0.595), fresh frozen plasma (16.79 units vs. 12.91 units, p=0.023, SMD 0.321), and platelets (8.76 units vs. 5.46 units, p=0.002, SMD 0.446) than the FC (+) group.
Conclusion:
The use of FC did not show survival benefits but reduced transfusion requirements in traumatic massive hemorrhages, highlighting a need for future investigations. In the future, individualized goal-directed transfusion with FC may play a significant role in treating massive bleeding.
PLyo après reconstitution ?
Post-Reconstitution Hemostatic Stability Profiles of Canadian and German Freeze-Dried Plasma
Henry T Peng H et Al. Life (Basel). 2024 Jan 24;14(2):172. doi: 10.3390/life14020172.
Despite the importance of the hemostatic properties of reconstituted freeze-dried plasma (FDP) for trauma resuscitation, few studies have been conducted to determine its post-reconstitution hemostatic stability. This study aimed to assess the short- (≤24 h) and long-term (≥168 h) hemostatic stabilities of Canadian and German freeze-dried plasma (CFDP and LyoPlas) after reconstitution and storage under different conditions. Post-reconstitution hemostatic profiles were determined using rotational thromboelastometry (ROTEM) and a Stago analyzer, as both are widely used as standard methods for assessing the quality of plasma. When compared to the initial reconstituted CFDP, there were no changes in ROTEM measurements for INTEM maximum clot firmness (MCF), EXTEM clotting time (CT) and MCF, and Stago measurements for prothrombin time (PT), partial thromboplastin time (PTT), D-dimer concentration, plasminogen, and protein C activities after storage at 4 °C for 24 h and room temperature (RT) (22-25 °C) for 4 h. However, an increase in INTEM CT and decreases in fibrinogen concentration, factors V and VIII, and protein S activities were observed after storage at 4 °C for 24 h, while an increase in factor V and decreases in antithrombin and protein S activities were seen after storage at RT for 4 h. Evaluation of the long-term stability of reconstituted LyoPlas showed decreased stability in both global and specific hemostatic profiles with increasing storage temperatures, particularly at 35 °C, where progressive changes in CT and MCF, PT, PTT, fibrinogen concentration, factor V, antithrombin, protein C, and protein S activities were seen even after storage for 4 h.
We confirmed the short-term stability of CFDP in global hemostatic properties after reconstitution and storage at RT, consistent with the shelf life of reconstituted LyoPlas. The long-term stability analyses suggest that the post-reconstitution hemostatic stability of FDP products would decrease over time with increasing storage temperature, with a significant loss of hemostatic functions at 35 °C compared to 22 °C or below. Therefore, the shelf life of reconstituted FDP should be recommended according to the storage temperature.
03/04/2023
Sang de banque sur pied: Mieux si on est entraîné
A prospective assessment of the medic autologous blood transfusion skills for field transfusion preparation
Steven G Schauer SG et Al. Transfusion. 2023 Mar 27. doi: 10.1111/trf.17325.
Background: Data demonstrate benefit from blood product administration near point-of-injury (POI). Fresh whole blood transfusion from a pre-screened donor provides a source of blood at the POI when resources are constrained. We captured transfusion skills data for medics performing autologous blood transfusion training.
Methods: We conducted a prospective, observational study of medics with varying levels of experience. Inexperienced medics were those with minimal or no reported experience learning the autologous transfusion procedures, versus reported experience among special operations medics. When available, medics were debriefed after the procedure for qualitative feedback. We followed them up to 7 days for adverse events.
Results: The median number of attempts for inexperienced and experienced medics was 1 versus 1 (interquartile range 1-1 for both, p=0.260). The inexperienced medics had a slower median time to needle venipuncture access for donation of 7.3 versus 1.5 minutes, needle removal after clamping time of 0.3 versus 0.2 minutes, time to bag preparation of 1.9 versus 1.0 minutes, time to IV access for reinfusion of 6.0 versus 3.0 minutes, time to transfusion completion of 17.3 versus 11.0 minutes, and time to IV removal of 0.9 versus 0.3 minutes (all p<0.05). We noted one administrative safety event in which allogeneic transfusion occurred. No major adverse events occurred. Qualitative data saturated around the need for quarterly training.
Conclusions: Inexperienced medics have longer procedure times when training autologous whole blood transfusion skills. This data will help establish training measures of performance for skills optimization when learning this procedure.
01/12/2022
Durée de conservation du sang en milieu hostile
Whole Blood Storage Temperature Investigation in Austere Environments
Cesar OA et Al. J Spec Oper Med. 2022 Sep 19;22(3):19-21.
Introduction:
Military medical research has affirmed that early administration of blood products and timely treatment save lives. The US Navy's Expeditionary Resuscitative Surgical System (ERSS) is a Role 2 Light Maneuver team that functions close to the point of injury, administering blood products and providing damage-control resuscitation and surgery. However, information is lacking on the logistical constraints regarding provisions for and the stability of blood products in austere environments.
Methods:
ERSS conducted a study on the United States Central Command (USCENTCOM) area of responsibility. Expired but properly stored units of stored whole blood (SWB) were subjected to five different storage conditions, including combinations of passive and active refrigeration. The SWB was monitored continuously, including for external ambient temperatures. The time for the SWB to rise above the threshold temperature was recorded.
Results:
The main outcome of the study was the time for the SWB to rise above the recommended storage temperature. Average ambient temperature during the experiment involving conditions 1 through 4 was 25.6°C (78.08°F). Average ambient temperature during the experiment involving condition 5 was 34.8°C (94.64°F). Blood temperature reached the 6°C (42.8°F) threshold within 90 minutes in conditions 1 and 2, which included control and chemically activated ice packs in the thermal insulated chamber (TIC). Condition 2 included prechilling the TIC in a standard refrigerator to 4°C (39.2°F), which kept the units of SWB below the threshold temperature for 490 minutes (approximately 8 hours). Condition 4 entailed prechilling the TIC in a standard freezer to 0.4°C (32.72°F), thus keeping the units of SWB below threshold for 2,160 minutes (i.e., 36 hours). Condition 5 consisted of prechilling the TIC to 3.9°C (39.02°F) in the combat blood refrigerator, which kept the SWB units below the threshold for 780 minutes (i.e., 13 hours), despite a higher average ambient temperature of almost +10°C (50°F).
Conclusion:
Combining active and passive refrigeration methods will increase the time before SWB rises above the threshold temperature. We demonstrate an adaptable approach of preserving blood product temperature despite refrigeration power failure in austere settings, thereby maintaining mission readiness to increase the survival of potential casualties.
France: Transfusion en Opex
An observational study of the blood use in combat casualties of the French Armed Forces, 2013–2021
Py N. et Al. Transfusion. 2022;1–14.
Background:
The French Armed Forces conduct asymmetric warfare in the Sahara-Sahel Strip. Casualties are treated with damage control resuscitation to the extent possible. Questions remain about the feasibility and sustainability of using blood for wider use in austere environments.
Methods:
We performed a retrospective analysis of all French military trauma patients transfused after injury in overseas military operations in Sahel-Saharan Strip, from the point of injury, until day 7, between January 11, 2013 to December 31, 2021.
Results:
Forty-five patients were transfused. Twenty-three (51%) of them required four red blood cells units (RBC) or more in the first 24H defining a severe hemorrhage. The median blood product consumption within the first 48 h, was 8 (IQR [3; 18]) units of blood products (BP) for all study population but up to 17 units (IQR [10; 27.5]) for the trauma patients with severe hemor- rhage. Transfusion started at prehospital stage for 20 patients (45%) and included several blood products: French lyophilized plasma, RBCs, and whole blood. Patients with severe hemorrhage required a median of 2 [IQR 0; 34] fur- ther units of BP from day 3 to day 7 after injury. Eight patients died in theater, 4 with severe hemorrhage and these 4 used an average of 12 products at Role 1 and 2.
Conclusion:
The transfusion needs were predominant in the first 48 h after the injury but also continued throughout the first week for the most severe trauma patients. Importantly, our study involved a low-intensity conflict, with a small number of injured combatants.
12/11/2022
Sang complet: OUI CHAUD et dans les 6 1ères heures.
Whole blood at the tip of the spear: A retrospective cohort analysis of warm fresh whole blood resuscitation vert: Dans les 06sus component therapy in severely injured combat casualties
Surgery . 2022 Feb;171(2):518-525. Gurney JM et Al.
Background
Methods
Results
Conclusion
21/10/2022
Plyo intraosseux ? Pas si simple
Intraosseous administration of freeze-dried plasma in the prehospital setting
Rittblat M et Al. Isr Med Assoc J. 2022 Sep;24(9):591-595.
Background:
Freeze dried plasma (FDP) is a commonly used replacement fluid in the prehospital setting when blood products are unavailable. It is normally administered via a peripheral intravenous (PIV) line. However, in severe casualties, when establishing a PIV is difficult, administration via intraosseous vascular access is a practical alternative, particularly under field conditions.
Objectives:
To evaluate the indications and success rate of intraosseous administration of FDP in casualties treated by the Israel Defense Forces (IDF).
Methods:
A retrospective analysis of data from the IDF-Trauma Registry was conducted. It included all casualties treated with FDP via intraosseous from 2013 to 2019 with additional data on the technical aspects of deployment collected from the caregivers of each case.
Results:
Of 7223 casualties treated during the study period, intravascular access was attempted in 1744; intraosseous in 87 of those. FDP via intraosseous was attempted in 15 (0.86% of all casualties requiring intravascular access). The complication rate was 73% (11/15 of casualties).
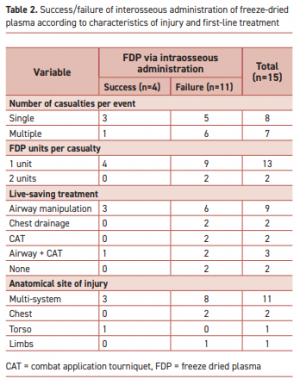
"According to the manufacturer’s specifications, the reconstituted lyophilized plasma product is delivered from a glass bottle with the rate of infusion determined primarily by gravity. This procedure precludes the care providers from applying external pressure to increase the plasma infusion rate. Therefore, the pressure gradient is determined primarily by gravity with respect to height of the bottle."
Complications were more frequent when the event included multiple casualties or when the injury included multiple organs. Of the 11 failed attempts, 5 were reported as due to slow flow of the FDP through the intraosseous apparatus. Complications in the remaining six were associated with deployment of the intraosseous device.
Conclusions:
Administration of FDP via intraosseous access in the field requires a high skill level.
| Tags : intraosseux
04/03/2022
Transfusion de sang frais
Use of Walking Blood Bank at Point of Injury During Combat Operations. A Case Report
Gaddy M et Al. J Spec Oper Med. Winter 2021;21(4):94-98
The US Military Tactical Combat Casualty Care guidelines recommend blood products as the preferred means of fluid resuscitation in trauma patients;, however, most combat units do not receive blood products prior to executing combat operations. This is largely due to logistical limitations in both blood supply and transfusion equipment. Further, the vast majority of medics are not trained in transfusion protocol. For many medics, the logistical constraints for cold-stored blood products favor the use of Walking Blood Bank (WBB), however few cases have been reported of WBB implementation at the point of injury during real world combat operations. This case report reviews one case of successful transfusion using WBB procedures at point of injury during combat. It highlights not only the feasibility, but also the necessity, for implementation of this practice on a larger scale.
| Tags : transfusion
20/01/2022
Fluid Resuscitation in Tactical Combat Casualty Care. Update 201
Fluid Resuscitation in Tactical Combat Casualty Care
TCCC Guidelines Change 21-01
4 November 2021
Dans ce document le mot important est choc hémorragique. Ce n'est pas parce q'un blessé saigne qu'il est en état de choc.
| Tags : choc, hémorragie, transfusion
17/07/2021
Transfuser avant l'hôpital: Pas suffisant pour réduire la mortalité
Effect of Prehospital Red Blood Cell Transfusion on Mortality and Time of Death in Civilian Trauma Patients
Background:
Current management principles of hemorrhagic shock after trauma emphasize earlier transfusion therapy to prevent dilution of clotting factors and correct coagulopathy. London's Air Ambulance (LAA) was the first UK civilian prehospital service to routinely offer prehospital red blood cell (RBC) transfusion (phRTx). We investigated the effect of phRTx on mortality.
Methods:
Retrospective trauma database study comparing mortality before implementation with after implementation of phRTx in exsanguinating trauma patients. Univariate logistic regression was performed for the unadjusted association between phRTx and mortality was performed, and multiple logistic regression adjusting for potential confounders.
Results:
We identified 623 subjects with suspected major hemorrhage. We excluded 84 (13.5%) patients due to missing data on survival status. Overall 187 (62.3%) patients died in the before phRTx period and 143 (59.8%) died in the after phRTx group. There was no significant improvement in overall survival after the introduction of phRTx (P = 0.554). Examination of prehospital mortality demonstrated 126 deaths in the pre-phRTx group (42.2%) and 66 deaths in the RBC administered group (27.6%). There was a significant reduction in prehospital mortality in the group who received RBC (P < 0.001).
Conclusions:
phRTx was associated with increased survival to hospital, but not overall survival. The “delay death” effect of phRTx carries an impetus to further develop inhospital strategies to improve survival in severely bleeding patients.
23/02/2021
Hypocalcémie ? Possible avant toute transfusion
Hypocalcemia in Military Casualties From Point of Injury to Surgical Teams in Afghanistan
Conner JR et Al. Mil Med . 2021 Jan 25;186(Suppl 1):300-304. doi: 10.1093/milmed/usaa267
---------------------------------------------------
On sait que les dérivés sanguins conservés en solution citratée exposent à une hypocalcémie lors de transfusions importantes, surtout en cas de traumatismes secondaires à des explosions. Apparemment cette dernière peut aussi survenir avant la mise en oeuvre de transfusion. C'est ce que laisse à penser ce document qui interpelle quand au rôle de la survenue d'une hypocalcémie (Ca2+ionisé) en phase préhospitalière. Quid de la validité des travaux ayant porté sur la transfusion hospitalière sans contrôle de ce paramètre Calcium ? Au TXA, faut il ajouter le Ca2+ ?
---------------------------------------------------
Hypocalcemia is a known sequela of citrated blood product transfusion. Civilian data suggest hypocalcemia on hospital admission is associated with worse outcomes. Initial calcium levels in military casualties have not previously been analyzed. The objective of this retrospective review aimed to assess the initial calcium levels in military trauma casualties at different Forward Surgical Teams (FST) locations in Afghanistan and describe the effects of prehospital blood product administration on arrival calcium levels.
This is a retrospective cohort analysis of military casualties arriving from point of injury to one of two FSTs in Afghanistan from August 2018 to February 2019 split into four locations. The primary outcome was incidence of hypocalcemia (ionized calcium < 1.20 mmol/L).
There were 101 patients included; 55 (54.5%) experienced hypocalcemia on arrival to the FST with a mean calcium of 1.16 mmol/L (95% confidence interval [CI], 1.14 to 1.18). The predominant mechanism of injury consisted of blast patterns, 46 (45.5%), which conferred an increased risk of hypocalcemia compared to all other patterns of injury (odds ratio = 2.42, P = .042).

Thirty-eight (37.6%) patients required blood product transfusion. Thirty-three (86.8%) of the patients requiring blood product transfusion were hypocalcemic on arrival. Mean initial calcium of patients receiving blood product was 1.13 mmol/L (95% CI, 1.08 to 1.18), which was significantly lower than those who did not require transfusion (P = .01). Eight (7.9%) of the patients received blood products before arrival, with 6/8 (75%) presenting with hypocalcemia.
Hypocalcemia develops rapidly in military casualties and is prevalent on admission even before transfusion of citrated blood products. Blast injuries may confer an increased risk of developing hypocalcemia. This data support earlier use of calcium supplementation during resuscitation.
15/11/2020
Solutés hypersalés: Sûrs pour la coagulation
The effect of hypertonic saline and mannitol on coagulation in moderate traumatic brain injury patients
Wang H et Al. Am J Emerg Med. 2017 Oct;35(10):1404-1407
-------------------------------------
Du moins pour le salé à 3%, administré ici pendant 3 jours.
-------------------------------------
Background:
Hyperosmolar therapy, using either hypertonic saline (HTS) or mannitol (MT), is considered the treatment of choice for intracranial hypertension, a disorder characterized by high intracranial pressure (ICP). However, hyperosmolar agents have been postulated to impair coagulation and platelet function. The aim of this study was to identify whether HTS and MT could affect coagulation in moderate traumatic brain injury (TBI) patients.
Methods: In this prospective and randomized double-blind study, we included adult patients with moderate TBI. Patients were divided into two groups according to the type of hypertonic solution administered. Group A patients received 20% MT and group B patients received 3% HTS. Rotational thromboelastometry (ROTEM) parameters were used to assess coagulation and platelet function.
Results:
ROTEM parameters included CT (clotting time), CFT (clot formation time), maximum clot firmness (MCF) measured by MCF (EXTEM and INTEM), MCF (FIBTEM) and standard coagulation tests (p>0.05). No significant differences were found between the two groups. Moreover, ROTEM parameters did not show significant changes at different time points after administration of the hyperosmolar solutions (p>0.05).
Conclusions: Overall, use of 3% HTS and 20% MT for the control of ICP did not significantly affect patients' coagulation function. Therefore, hyperosmotic solution is safe and does not increase the risk of intracranial rebleeding.
11/11/2020
Inhibition de l'histone déacetylsae pour l'hémorragie
Histone deacetylase 6 inhibition improves survival in a swine model of lethal hemorrhage, polytrauma, and bacteremia
Biesterveld BE et Al. J Trauma Acute Care Surg . 2020 Nov;89(5):932-939.
---------------------
Le TXA fait partie de l'arsenal thérapeutique en cas d'hémorragie sévère d'origine traumatique. Cela pourrait être également le cas des inhibiteurs de l'histone déacétylsae, famille de l'acide valproïque - Dépakine-
---------------------
Background: Trauma is the leading cause of death for young Americans. Nonspecific histone deacetylase inhibitors, such as valproic acid, have been shown to improve survival in preclinical models of lethal trauma, hemorrhage, and sepsis. The doses needed to achieve a survival benefit are higher than Food and Drug Administration-approved doses, and the nonspecificity raises concerns about unintended adverse effects. The isoform-specific histone deacetylase 6 inhibitor, ACY-1083, has been found to be as efficacious as valproic acid in a rodent model of hemorrhagic shock. We hypothesized that ACY-1083 treatment would improve survival in a swine model of lethal hemorrhage, polytrauma, and bacteremia.
Methods: Swine were subjected to 45% blood volume hemorrhage, brain injury, femur fracture, rectus crush, splenic and liver lacerations, and colon injury. After 1 hour of shock (mean arterial pressure, 30-35 mm Hg), animals were randomized to normal saline resuscitation (control) or normal saline plus ACY-1083 30 mg/kg treatment (n = 5/group). After 3 hours (simulating delayed evacuation), packed red blood cells and antibiotics were administered, the colon injury was repaired, and the abdomen was closed. Animals were then monitored for another 4 hours. Survival was assessed using Kaplan-Meier and log-rank test.
Results: This combination of injuries was lethal. All animals became bacteremic, in addition to the severe hemorrhagic shock. Survival in the control group was 0%, and ACY-1083 treatment increased survival to 80% (p = 0.019). There was no difference in the brain lesion size between the groups.
Conclusion: A single dose of ACY-1083 markedly improves survival in an otherwise lethal model of polytrauma, hemorrhagic shock, and bacteremia.
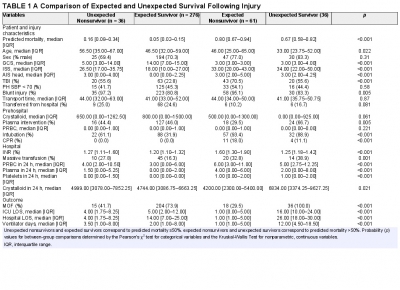
Plasma préhospitalier. Surtout pour certains
Characterization of unexpected survivors following a prehospital plasma randomized trial
J Trauma Acute Care Surg. 2020 Nov;89(5):908-914
Background: Prehospital plasma improves survival for severely injured trauma patients transported by air ambulance. We sought to characterize the unexpected survivors, patients who survived despite having high predicted mortality after traumatic injury.
Methods: The Prehospital Air Medical Plasma trial randomized severely injured patients (n = 501) to receive either standard care (crystalloid) or two units of prehospital plasma followed by standard care fluid resuscitation. We built a generalized linear model to estimate patient mortality. Area under the receiver operating characteristic curve was used to evaluate model performance. We defined unexpected survivors as patients who had a predicted mortality greater than 50% and survived to 30 days. We characterized patient demographics, clinical features, and outcomes of the unexpected survivors. Observed to expected (O/E) ratios and Z-statistics were calculated using model-estimated mortality for each cohort.
Results: Our model predicted mortality better than Injury Severity Score or Revised Trauma Score parameters and identified 36 unexpected survivors. Compared with expected survivors, unexpected survivors were younger (33 years [24, 52 years] vs. 47 years [32, 59 years], p = 0.013), were more severely injured (Injury Severity Score 34 [22, 50] vs. 18 [10, 27], p < 0.001), had worse organ dysfunction and hospital resource outcomes (multiple organ failure, intensive care unit, hospital length of stay, and ventilator days), and were more likely to receive prehospital plasma (67 vs. 46%, p = 0.031).
Nonsurvivors with high predicted mortality were more likely to receive standard care resuscitation (p < 0.001). Unexpected survivors who received prehospital plasma had a lower observed to expected mortality than those that received standard care resuscitation (O/E 0.56 [0.33-0.84] vs. 1.0 [0.73-1.32]). The number of prehospital plasma survivors (24) exceeded the number of predicted survivors (n = 10) estimated by our model (p < 0.001).
Conclusion: Prehospital plasma is associated with an increase in the number of unexpected survivors following severe traumatic injury. Prehospital interventions may improve the probability of survival for injured patients with high predicted mortality based on early injury characteristics, vital signs, and resuscitation measures.
22/10/2020
Albumine: Le retour ?
Should Albumin be Considered for Prehospital Resuscitation in Austere Environments? A Prospective Randomized Survival Study in Rabbits
Kheirabadi BS et Al. Shock . 2020 Sep;54(3):358-367.
--------------------------------
Le remplissage vasculaire par albumine diluée n'est pas spécialement recommandé. Pourtant il semblerait que cela soit à tort dans certains environnements. C'est du moins ce que laisse penser ce travail expérimental chez le lapin.
--------------------------------
Background:
The new guidelines for prehospital care of combat casualties in shock recommend administration of whole blood or blood components to increase blood pressure to a permissible hypotensive level (i.e., hypotensive resuscitation [HR]). We investigated if 2 h of HR using limited volumes of whole blood, plasma, or albumin would lead to full recovery and long-term survival of rabbits subjected to severe hemorrhagic shock (HS).
Methods:
Following instrumentation, laparotomy was performed on IV-anesthetized spontaneously breathing New Zealand white rabbits (3.0 kg -3.5 kg). Next, ∼40% of rabbits' blood volume was removed producing HS (mean arterial pressure [MAP]∼20 mm Hg). Fifteen minutes later, rabbits were resuscitated with a limited volume (12.5 mL/kg) of rabbit whole blood (fresh whole blood [FWB]), rabbit fresh frozen plasma (FFP), or 5% human albumin (ALB) to a target pressure (MAP) of 60 mm Hg (n=8/grp) and monitored for 2 h. Liver bleeding time was measured at baseline and 10 min after HR. Subsequently, animals were fully resuscitated (blood + lactated Ringer [LR]), surgically repaired, and recovered for 8 days. An untreated group (n = 6) was also included.
Results:
Following HS, lactate and base deficit levels were increased to 8.2 ± 1.6 and 12.9 ± 3.1 mM respectively with no difference among groups. A lower volume of FWB volume was required to reach the target MAP (P < 0.05 vs. ALB) but MAP declined during the HR period (P < 0.01 vs. ALB).
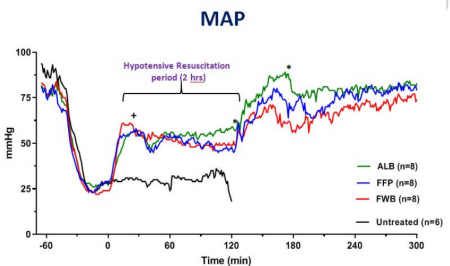
FWB provided higher hematocrit and platelets but it did not reduce lactate level faster than other fluids. Beside higher fibrinogen, no differences were found in hemostatic or resuscitative effects of FFP versus ALB. Bleeding time was prolonged with ALB and FFP fluids but unchanged with FWB. Untreated rabbits died during shock or shortly after. All treated rabbits except one recovered and lived for 8 days with normal blood tests and similar tissue histology.
Conclusions:
Two hours of HR using a limited volume of FWB, FFP, or ALB led to full recovery and long-term survival of rabbits subjected to HS. Apart from bleeding time, no clinically significant differences were found among the three fluids. Five percent human albumin solutions are isotonic, iso-oncotic, ready-to-use, stable, and compatible with all blood types and should be considered for prehospital resuscitation where blood products are not available or not accepted.
| Tags : remplissage
02/01/2020
Le fibrinogène se conserve bien au chaud comme au froid
Stability of Fibrinogen Concentrate in Human Blood Samples: An In Vitro Study
Objectives:
This study was designed to assess the stability and functional activity of fibrinogen concentrates subjected to the changes in temperature and duration observed in field conditions.
Methods:
Fibrinogen concentrate was stored at -20°C (12 vials), 22°C (12 vials), and 50°C with 80% humidity (12 vials), for up to 6 mo. At each temperature, three vials of fibrinogen concentrate were taken out at 0, 1, 3, and 6 mo and reconstituted. On analysis days, blood samples were taken from a single healthy donor to collect plasma samples. The donor plasma was mixed with commercial fibrinogen-deficient plasma to make fibrinogen-adjusted plasma (FAP). An aliquot of the reconstituted fibrinogen concentrate was used for quantification of stored fibrinogen content (using STA-R) and function (Rotem - Fibtem) in FAP.
Results:
At 22°C for 0, 1, 3, and 6 mo, there were no significant changes observed in fibrinogen content (1,223 ± 42 mg/vial, 1,286 ± 86 mg/vial, 1,234 ± 76 mg/vial, and 1,178 ± 64 mg/vial), prothrombin time (13.5 ± 0.1 s, 13.7 ± 0.6 s, 13.3 ± 0.4 s, and 13.7 ± 0.2 s), or activated partial thromboplastin time (31.1 ± 0.2 s, 32.0 ± 0.2 s, 31.5 ± 0.2 s, and 32.0 ± 0.8 s), respectively. There were also no significant changes observed in any of the Fibtem measurements. Similarly, no differences were observed in these variables over time at -20°C and 50°C with 80% humidity.
Conclusions:
Fibrinogen concentrate maintained its content and function when stored at -20°C to 50°C with up to 80% humidity for 6 mo.
| Tags : fibrinogène
04/12/2019
HEA 130: Protègerait le glycocalyx ?
The protective effect of hydroxyethyl starch solution on the glycocalyx layer in an acute hemorrhage mouse model.
- --------------------------------------------------------
Le recours au HEA pour le remplissage vasculaire est très décrié notamment à cause d'effets rénaux délétères observés tout particulièrement en cas de sepsis. Les HEA peuvent néanmoins être utilisés en cas de non restauration de l'hémodynamique après emploi de cristalloides isotoniques et en attendant les produits de transfusion (reco 6 de la RFE sur le choc hémorragique). Ce travail semble donc tempérer un peu le rejet des HEA pour la réanimation du choc hémorragique. Il montre une moindre élévation de syndecan-1 lors de l'emploi d'HEA 130 qu'après Salé.
--------------------------------------------------------
PURPOSE:
Fluid therapy focused on glycocalyx (GCX) protection in hemorrhagic shock is a current focus of research. Hydroxyethyl starch (HES) solution is commonly used for fluid resuscitation; however, its effects on the GCX remain unclear. The primary aim of this study was to explore the protective effect of HES130 in maintaining GCX thickness and reducing plasma syndecan-1 expression.
METHODS:
An acute hemorrhage murine model with the dorsal skin chambers was used to measure GCX thickness and to evaluate vascular permeability. Groups of mice were treated with normal saline (NS), albumin (NS-A), HES130 (NS-V), or no exsanguination or infusion (C). We measured syndecan-1 plasma concentrations, performed blood gas analysis, and analyzed the 7-day cumulative mortality.
RESULTS:
GCX thickness in NS mice was significantly reduced compared to that in group C, but no other groups showed a difference compared to group C. The plasma concentration of syndecan-1 was significantly higher in NS mice than in group C. There were no significant differences in the fluorescence intensity of dextran in the interstitial space. HES70 leakage was suppressed in NS-V mice compared to those in other groups. HES70 was localized to the inner vessel wall in C, NS, and NS-A mice, but not in group NS-V. Blood gas analysis indicated that pH and lactate showed the greatest improvements in NS-V mice. The 7-day cumulative mortality rate was the highest in group NS.
CONCLUSION:
Resuscitation with HES130 protected the GCX and suppressed vascular permeability of HES70 during early stages of acute massive hemorrhage.