11/11/2014
Videolaryngoscope en préhospitalier ? Pas si évident !
What is the role of video laryngoscopy in prehospital care ?
Voelckel WG et Al. Scand J Trauma Resusc Emerg Med. 2014; 22(Suppl 1): A6
Une réflexion qui exprime, malgré un apprentissage plus rapide et une meilleure vision, le manque actuel de données sur l'intérêt de la vidéolaryngoscopie préhospitalière. En effet meilleure vision ne signifie pas insertion plus facile du tube trachéal dans la trachée (lire ces argumentaires: 1, 2, 3, 4). Les auteurs lui prédisent cependant une place importante dans les années à venir.et en attendant:
APPRENEZ A INTUBER EN LARYNGOSCOPIE DIRECTE ET MAINTENEZ VOTRE SAVOIR FAIRE
| Tags : airway
07/11/2014
Lidocaïne intraarticulaire: A ne pas oublier
Intra-articular lidocaine versus intravenous analgesia and sedation for manual closed reduction of acute anterior shoulder dislocation: an updated meta-analysis
Jiang N et Al. J Clin Anesth. 2014 Aug;26(5):350-9
--------------------------------------------------------------------------
Une révision de la revue cochrane de 2011. La lidocaïne intraveineuse est efficace plus rapidement (attendre 5 minutes avant réduction) mais a pour inconvénient d'avoir plus de complications que lors d'une injection intraarticulaire (attendre au moins 15 minutes avant réduction)
--------------------------------------------------------------------------
STUDY OBJECTIVE:
To compare intra-articular lidocaine (IAL) with intravenous analgesia and sedation (IVAS) for manual closed reduction of acute anterior shoulder dislocation.
DESIGN:
Meta-analysis.
SETTING:
Metropolitan medical university.
MEASUREMENTS:
A literature search was conducted of PubMed, Ovid and Cochrane Library, to identify randomized controlled trials (RCTs) published from January 1, 1990 to September 1, 2012, that compared IAL with IVAS for manual closed reduction of acute anterior shoulder dislocation. Effective data were pooled using fixed-effects or random-effects models with mean differences (MDs) and risk ratios (RRs) for continuous and dichotomous variables, respectively.
MAIN RESULTS:
Nine RCTs comprising 438 patients were analyzed. Statistical analyses showed that IAL was superior to IVAS with respect to lower complication risk (P < 0.00001) and shorter mean hospital length of stay (P = 0.03). No significant differences were noted in success of joint reduction (P = 0.16), patient satisfaction (P = 0.12), or postreduction pain relief (P = 0.76). However, IAL required more time than IVAS from injection to reduction (P < 0.00001). Subgroup analyses showed that IVAS was associated with higher risks of respiratory depression (P < 0.0001), vomiting (P = 0.04), and thrombophlebitis (P = 0.008), but no statistical differences were identified in nausea (P = 0.06), hypotension (P = 0.10), drowsiness (P = 0.45), or headache (P = 0.29).
CONCLUSIONS:
Intra-articular lidocaine injection may be safer than IVAS because there are fewer risks of postoperative complications with IAL. Both techniques are similarly effective for manual closed reduction of acute anterior shoulder dislocation.
05/11/2014
Heatpac: Ancien, oublié mais reste d'actualité
Pre-hospital torso-warming modalities for severe hypothermia: a comparative study using a human model
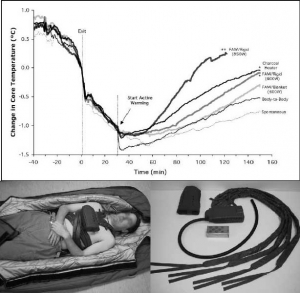
Objective: To compare 5 active torso-warming modalities in a human model of severe hypothermia with shivering heat production inhibited by intravenous meperidine.
Methods: Six subjects were cooled on 6 different occasions each, in 8°C water, for 30 minutes or to a core temperature of 35°C. Spontaneous warming was the first torso-warming modality to be tested for every subject, and results served both as a comparative control and for determination of the meperidine dose for subsequent trials. Meperidine (1.5 mg/kg) was administered during the final 10 minutes of immersion to suppress shivering. Subjects were removed from the water, dried and insulated for 30 minutes, followed by 120 minutes of 1) forced-air warming with either a 600-W heater and commercial soft warming blanket; or 2) a 600-W heater and rigid cover; or 3) an 850-W heater and rigid cover; or 4) a charcoal heater on the chest; or 5) direct body-to-body contact with a normothermic partner. Supplemental meperidine (to a maximum cumulative dose of 3.2 mg/kg) was administered as required to inhibit shivering.
Results: The initial post-cooling afterdrop was approximately 1.0°C. After 30 minutes, core temperature continued to drop by 0.45°C in spontaneous and body-to-body warming modalities. This post-warming afterdrop was significantly less with 600-W heater and rigid cover and the charcoal heater (0.26°C) and the least with 850-W heater and rigid cover (0.17°C). Core rewarming rates were highest using 850-W heater and rigid cover (1.45°C/hr), with charcoal heating and 600-W rigid heater (0.7°C/hr), 600-W heater and blanket (0.57°C/hr) and body-to-body warming (0.52°C/hr) being more effective than spontaneous warming (0.36°C/hr).
Conclusions: In non-shivering subjects, external heat application was effective in attenuating core temperature afterdrop and facilitating safe core rewarming; this was more evident when heat was delivered preferentially to the chest, and dependent upon the amount of heat donated. The modalities studied appear sufficiently practical and portable for pre-hospital use and should be considered for such situations, particularly in rural or wilderness locations where anticipated transport time to the hospital exceeds 30 minutes.
| Tags : hypothermie
04/11/2014
Gelures: L'expérience indienne
Management of Cold Injuries
Hota PK et Al. Surgical Research Updates, 2013, 1, 20-25
Un article produit par une équipe ayant une grande connaissance de cette pathologie. La place de l'oxygénothérapie hyperbare y est mise en avant.
____________________________________________________________
____________________________________________________________
| Tags : gelures
02/11/2014
Biomed Central Free Online Full-text Articles
Highwire Free Online Full-text Articles
01/11/2014
Garrot: A partir de quand est on fiable ?
Single versus Double Routing of the Band in the Combat Application Tourniquet.
J Spec Oper Med. 2013 Spring;13(1):34-41.
Le sauvetage au combat met en avant l'importance d'arrêter toute hémorragie le plus tôt possible. Le garrot tient là une place essentielle. Mais à partir de quand l'apprentissage de cette technique peut il être considéré comme optimal. Une réponse est apporté par ce travail: La maîtrise de ce geste semble être obtenue à partir de 30 poses.
Background: Common first aid tourniquets, like the Combat Application Tourniquet (CAT) of a windlass and band design, can have the band routed through the buckle in three different ways, and recent evidence indicates users may be confused with complex doctrine.
Objective: The purpose of the present study is to measure the differential performance of the three possible routings in order to better understand good tourniquet practice.
Methods: A training manikin was used by two investigators to measure tourniquet effectiveness, time to stop bleeding, and blood loss.
Results: The effectiveness rate was 99.6% (239/240) overall. Results were similar for both single-slit routings (inside vs. outside, p > 0.05). Effectiveness rates (yes-no results for hemorrhage control expressed as a proportion of iterations) were not statistically different between single and double routing. However, the time to stop bleeding and blood loss were statistically different (p < 0.05).
Conclusions: CAT band routing, through the buckle either singly or doubly, affects two key performance criteria: time to stop bleeding and volume of blood lost. Single routing proved to be faster, thereby saving more blood. Learning curves required to optimize user performance varied over 30-fold depending on which variable was selected (e.g., effectiveness vs. blood loss).
| Tags : tourniquet
N'oublions pas: Comprimer est essentiel
Laboratory assessment of out-of-hospital interventions to control junctional bleeding from the groin in a manikin model l.
Kragh JF et Al Am J Emerg Med. 2013 Aug;31(8):1276-8
Junctional body regions between the trunk and its appendages, such as the groin, are too proximal for a regular limb tourniquet to fit [1,2]. Not since 1993’s Black Hawk Down has junctional hemorrhage control become such a hot topic in military casualty care [1–7]. In February 2013, the US military’s Task Force Medical Afghanistan requested a fill of a gap in junctional hemorrhage control as an urgent operational need, meaning that junctional hemorrhage control devices should be considered urgently to fill a gap in medical care in war. A small but growing body of evidence indicates that hemorrhage control can be attained out-of-hospital with mechanical compression, using such interventions as medical devices, on a pressure point proximal to a bleeding wound [3–9]. To evaluate laboratory use of junctional hemorrhage control interventions, we gathered data on stopping groin bleeding in a manikin model to understand the plausibility of such interventions for future human subject research.
Under an approved protocol, we tested efficacy of interventions in a manikin designed to train medics in out-of-hospital hemorrhage control (Combat Ready Clamp [CRoC] Trainer Manikin, Operative Experience, Inc, North East, MD). We filled the blood reservoir with 4 liters of water; we refilled the reservoir after 5 iterations or 1.5 liters of lost fluid, whichever came first. The manikin had a right-groin gunshot wound through the proximal thigh where the common femoral artery flow was controllable by skin compression over it at the level of the inguinal fold. There was 3 cm between the pressure point where compression was applied and the proximal extent of the wound. Interventions were timed, blood loss was measured, and efficacy was noted. Efficacy was operationally defined as visually stopped flow into the wound from the vessel lumen. Pearls and pitfalls of intervention use were recorded.
Interventions to control hemorrhage included medical device use, manual or digital compression, and improvised use of a rock-like kettlebell (to simulate a rock used in care on the battlefield in a case recorded in the Department of Defense Trauma Registry in 2012). Interventions included digital (finger) compression, manual compression (heel of the hand), knee compression, compression by a 50lb kettlebell (Hampton Fitness Products, Ventura, CA), and medical device use (Combat Ready Clamp, CRoC, Combat Medical Systems, Fayetteville, NC; SAM Junctional Tourniquet, SAM, SAM Medical Products, Portland, OR; Junctional Emergency Treatment Tool, JETT, North American Rescue Products, Greer, SC; Abdominal Aortic Tourniquet, AAT, Compression Works, Hoover, AL). The first device assessed was the CRoC which, of the devices studied, was cleared first by the US Food and Drug Administration on August 11, 2010. The first setting of the evaluation (which was for the CRoC) was in a simulation center as previously reported with three to five people, and the other setting of the evaluation was on a table with one to three people [5]. The data from that initial setting is included here for comparison of time to stop bleeding, blood loss volume, and device efficacy [5]. Since the blood loss rate was non-linear (as it is in real situations for casualties because bleeding is brisker initially rather than later), we did not refill the bladder after each iteration. The manikin was not designed to differentiate between performance of devices, so we only compared results to acceptable benchmarks. The benchmark for time to stop bleeding was 300 seconds (s), and the benchmark for blood loss was a normal adult male blood volume, 5 L. Hemorrhage was controlled with 100% efficacy in the manikin model for each intervention. The times to stop bleeding and volumes of blood lost were acceptable for all devices and iterations (Figs. 1 and 2; Tables 1 and 2). Advantages and disadvantages were learned with experience in the use of each intervention (Table 3). Traits of interventions varied through wide ranges (Table 4).
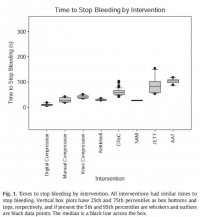
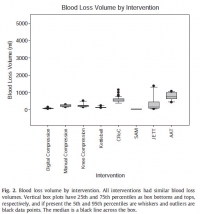
N'oublions pas le packing de plaie
A manikin model for study of wound-packing interventions to control out-of-hospital hemorrhage
Kragh JF et Al. Am J Emerg Med. 2014 Sep;32(9):1130-1
To the Editor,
With hemorrhage being the primary cause of mortality on the battlefield [1-3], wound-packing practice by US military medics in the wars since September 11, 2001, has changed from a conservative to an assertive approach. The foremost emphasis changed from preventing contamination to controlling hemorrhage. As no specific hemostatic dressings were available at the start of the wars, after such dressings were fielded, medics changed their approach by packing wounds with more gauze earlier in casualty care and deeper into subfascial cavitary wounds as a way to control hemorrhage. Although experienced medics and trainers favor an assertive approach, there is limited empirical evidence of improvements. In addition, with the development of various dressings with hemostatic properties [4-6], no systematic approach to trial wound-packing techniques easily has been developed. When a war ends and military medical care shifts toward peacetime duties and garrison work, skill sets in trauma care degrade as skill performance is less often. Furthermore, peacetime training reverts back toward everyday work such as sick call and away from future combat casualty care. The reversion tendency allows less training of new medics in combat casualty care than those who were trained during busy years of sustained combat; like nothing in peacetime, the present danger of combat during wartime focuses attention on hemorrhage control. A challenge for medics to be as well trained in peacetime in combat casualty care as during wartime is a recurring theme of military medicine.
Of the medical advances in prehospital combat casualty care during the current wars, we feel that the most important are regular tourniquets, junctional tourniquets, and wound packing because of their potential capacity to save numerous casualties from the most common cause of death on the battlefield—wound exsanguination. To not backslide on these 3 skills, we continue scholarly work to refine them. We call these skills the “Big 3,” and we have published mostly on tourniquets. To stimulate development of best practices in wound packing, we now focus the present report on an introductory test method to increase awareness of knowledge gaps within the science of wound packing.
The purpose of the present study is to introduce a laboratory model of hemorrhage with data comparing gauze wound packing and medical device use to better understand out-of-hospital hemorrhage control. In an approved protocol, we used a manikin model designed for the capacity to train medics in techniques of gauze wound packing for hemorrhage control in trauma care. The manikin trainer (Combat Ready Clamp [CRoC] Trainer Manikin; Operative Experience, Inc, North East, MD) had a gunshot wound of the right groin that bled water from the common femoral artery; the wound track went through the thigh posterior to anterior. We measured blood volume lost from bleeding, the application time, and hemorrhage control (yes-no). We had only 1 user who had never packed a wound prehospital and had never been trained in this task. We made 4 tests. The first test was that we used a type of gauze (QuikClot Combat Gauze; Z-Medica, Wallingford, CT) alone in accordance with its instructions for use (IFU) except we used no overwrap for pressure; the overwrap is the fourth and final step of the gauze IFU. The second test was like the first, but we used the full IFU that included use of an overwrap (AirWrap, RevMedx, Wilsonville, OR). The third test was only the A manikin model for study of wound-packing interventions to control out-of-hospital hemorrhage
use of the overwrap and no gauze; this test included no inflation of the pneumatic bladder within the overwrap. The fourth test included the gauze, the overwrap, and the inflation of the overwrap. Each test had 4 replicates.
The results showed an apparent differential performance of the methods of hemorrhage control, but the test order indicated possible learning that may be a confounder. The fourth test performed better than the first 3 with respect to reduced blood loss (Fig. 1), which may mean that the fourth method is best or that the user learned with experience. Perhaps both are true. Application time also improved (Fig. 2), and the evaluation was sensitive enough to detect longer application times with additional steps in the wound-wrapping process.
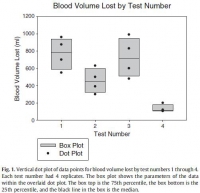
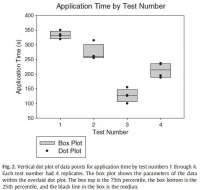 

There was no surprise that the gauze performed better as indicated by reduced blood loss (Fig. 1) when it was used fully in accordance with its IFU in that the second test performed better than the first. In addition, there was no surprise that the overwrap performed better when it was used fully in accordance with its intent in that the fourth test performed better than the third since the overwrap was designed to wrap over gauze.
The strength of this report is that it introduces a method of analyzing wound-packing techniques that generated hypotheses for testing. Hypothesis-driven experiments will follow this hypothesis generating report to check differential performance of techniques such as preliminarily studied here. The method permits learning curve analysis to see how fast users can be in hemorrhage control; we have previously found that tourniquet use, for example, appears to take more than 30 tests before users flatten their learning curve [7].
Determining optimal care techniques and training regimens may help to improve clinical performance. The limitation of the present report is its introductory design; the preliminary finding is only able to generate hypotheses. Future directions include analyses of techniques and learning curves.
John F. Kragh Jr., MD
US Army Institute of Surgical Research
Joint Base San Antonio
Fort Sam Houston, TX
Uniformed Services University of the Health Sciences
F. Edward Hébert School of Medicine Bethesda, MD
Corresponding author at: US Army Institute of Surgical Research
Damage Control Resuscitation, 3698 Chambers Pass
Ste B, Joint Base San Antonio
Fort Sam Houston, TX
E-mail address: john.f.kragh.civ@mail.mil
John Steinbaugh
RevMedx, Inc, Wilsonville, OR
Donald L. Parsons, PA-C
Combat Medic Training
US Army Medical Department Center and School
Joint Base San Antonio, Fort Sam Houston, TX
Robert L. Mabry, MD
Emergency Medical Services Fellowship
San Antonio Military Medical Center
Joint Base San Antonio
Fort Sam Houston, TX
Bijan S. Kheirabadi, PhD
Michael A. Dubick, PhD
Damage Control Resuscitation
US Army Institute of Surgical Research
Joint Base San Antonio
Fort Sam Houston, TX
| Tags : packing


