13/12/2025
Ketamine intranasale: Bon pour le service ?
Breathing new life into pain management: a systematic review of nebulised ketamine for analgesia
Daniel Kirk D et Al. Scand J Trauma Resusc Emerg Med. 2025 Dec 4;33(1):196.
Background:
Acute pain accounts for 60-90% of presentations to the emergency department (ED), with 20-40% of patients reporting severe pain. Current management practices, including simple analgesics, opiates and anti-inflammatory drugs, are often inadequate or slow to reach peak effect, necessitating the exploration of alternative analgesics. Ketamine, acting primarily through N-methyl-D-aspartate (NMDA) receptor antagonism, presents a promising alternative due to its rapid onset. However, its nebulised form remains underutilised in clinical practice.
Aims and objectives:
This review evaluates the efficacy of nebulised ketamine in reducing pain in adult ED patients, alongside its side effect profile, optimal dosing, and potential as an alternative or adjunctive analgesic compared to other treatments.
Methods and design:
A systematic review utilising the PRISMA guidelines was conducted. Searches were carried out in Medline, Embase, PubMed, Science Direct, google scholar and Ovid databases from 2010 to May 2024, including studies containing objective analysis of pain control with nebulised ketamine. A two-sample t-test was used to assess statistical significance. Quality assessment was performed using the CASP tool, and bias was evaluated using the ROBINS-I and ROB2 tool.
Results:
Of 99 articles, 9 (5 randomised controlled trials, 3 case series and 1 case report) totalling 453 patients were included. All studies suggested improvement in pain scores with nebulised ketamine, with an average reduction of 42.5% and 70.4% over a 15 and 120-minute period respectively (p < 0.0001). Higher doses (1 mg/kg, 1.5 mg/kg) did not significantly improve pain compared to lower doses (0.7 mg/kg 0.75 mg/kg), with similar overall reductions reported across all four dosing regimens (p < 0.0003 or 0.0001).
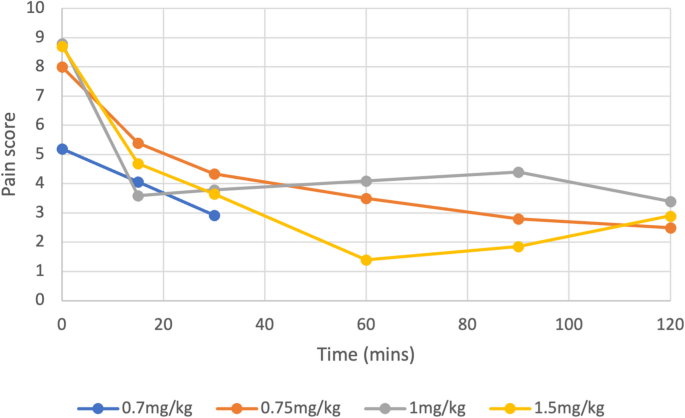
Nebulised ketamine was non-inferior to intravenous (IV) morphine, IV ketamine, nebulised dexmedetomidine, and Entonox, and had fewer side effects.
Conclusion: Nebulised ketamine offers a viable alternative for pain management in emergency settings, providing effective analgesia with a favourable safety profile. Further multicentre trials with larger populations are recommended to confirm these findings and establish standardised dosing protocols for consideration in national guidance.
09/10/2024
Analgésie par inhalation ? Pas un plébiscite
Inhaled analgesics for the treatment of prehospital acute pain—A systematic review
Hyldmo PK et Al. Acta Anaesthesiol Scand. 2024 Sep 26. doi: 10.1111/aas.1452
Background
Many prehospital emergency patients receive suboptimal treatment for their moderate to severe pain. Various factors may contribute. We aim to systematically review literature pertaining to prehospital emergency adult patients with acute pain and the pain-reducing effects, adverse events (AEs), and safety issues associated with inhaled analgetic agents compared with other prehospital analgesic agents.
Methods
As part of an initiative from the Scandinavian Society of Anaesthesia and Intensive Care Medicine, we conducted a systematic review (PROSPERO CRD42018114399), applying the PRISMA guidelines, Grading of Recommendations Assessment, Development, and Evaluation (GRADE), and Cochrane methods, searching the Cochrane Library, Epistemonikos, Centre for Reviews and Dissemination, PubMed, and EMBASE databases (updated March 2024). Inclusion criteria were the use of inhaled analgesic agents in adult patients with acute pain in the prehospital emergency care setting. All steps were performed by minimum of two individual researchers. The primary outcome was pain reduction; secondary outcomes were speed of onset, duration of effect, and relevant AEs.
Results
We included seven studies (56,535 patients in total) that compared inhaled agents (methoxyflurane [MF] and nitrous oxide [N2O]) to other drugs or placebo. Study designs were randomized controlled trial (1; n = 60), randomized non-blinded study (1; n = 343), and randomized open-label study (1; n = 270). The remaining were prospective or retrospective observational studies. The evidence according to GRADE was of low or very low quality. No combined meta-analysis was possible. N2O may reduce pain compared to placebo, but not compared to intravenous (IV) paracetamol, and may be less effective compared to morphine and MF. MF may reduce pain compared to paracetamol, ketoprofen, tramadol, and fentanyl. Both agents may be associated with marked but primarily mild AEs.
Conclusion
We found low-quality evidence suggesting that both MF and N2O are safe and may have a role in the management of pain in the prehospital setting. There is low-quality evidence to support MF as a short-acting single analgesic or as a bridge to IV access and the administration of other analgesics. There may be occupational health issues regarding the prehospital use of N2O.
14/02/2022
Kétamine: Stable longtemps quand il fait très chaud
Ketamine Stability over Six Months of Exposure to Moderate and High Temperature Environments
Foertsch MJ et Al. Prehosp Emerg Care. 2021 Jun 21;1-6.
Background:
All medications should be stored within temperature ranges defined by manufacturers, but logistical and operational challenges of prehospital and military settings complicate adherence to these recommendations. Lorazepam and succinylcholine experience clinically relevant heat-related degradation, whereas midazolam does not. Because ketamine's stability when stored outside manufacturer recommendations is unknown, we evaluated the heat-related degradation of ketamine exposed to several temperature ranges.
Methods: One hundred twenty vials of ketamine (50 mg/mL labeled concentration) from the same manufacturer lot were equally distributed and stored for six months in five environments: an active EMS unit in southwest Ohio (May-October 2019); heat chamber at constant 120 °F (C1); heat chamber fluctuating over 24 hours from 86 °F-120 °F (C2); heat chamber fluctuating over 24 hours from 40 °F-120 °F (C3); heat chamber kept at constant 70 °F (manufacturer recommended room temperature, C4). Four ketamine vials were removed every 30 days from each environment and sent to an FDA-accredited commercial lab for high performance liquid chromatography testing. Data loggers and thermistors allowed temperature recording every minute for all environments. Cumulative heat exposure was quantified by mean kinetic temperature (MKT), which accounts for additional heat-stress over time caused by temperature fluctuations and is a superior measure than simple ambient temperature. MKT was calculated for each environment at the time of ketamine removal. Descriptive statistics were used to describe the concentration changes at each time point.
Results: The MKT ranged from 73.6 °F-80.7 °F in the active EMS unit and stayed constant for each chamber (C1 MKT: 120 °F, C2 MKT: 107.3 °F, C3 MKT: 96.5 °F, C4 MKT: 70 °F). No significant absolute ketamine degradation, or trends in degradation, occurred in any environment at any time point. The lowest median concentration occurred in the EMS-stored samples removed after 6 months [48.2 mg/mL (47.75, 48.35)], or 96.4% relative strength to labeled concentration.
Conclusion: Ketamine samples exhibited limited degradation after 6 months of exposure to real world and simulated extreme high temperature environments exceeding manufacturer recommendations. Future studies are necessary to evaluate ketamine stability beyond 6 months.
| Tags : kétamine
09/02/2022
Efficacité et sécurité de la kétamine pour l'analgésie préhospitalière du blessé de guerre
07/12/2021
Kétamine préhospitalière: Reco US
| Tags : kétamine
09/07/2021
Kétamine: Pas assez utilisée en role 1 ?
Ketamine Use in Operation Enduring Freedom
Eric L. et Al. Mil Med. 2021 Jul 1;186(7-8):e720-e725
Introduction:
Ketamine is a dissociative anesthetic increasingly used in the prehospital and battlefield environment. As an analgesic, it has been shown to have comparable effects to opioids. In 2012, the Defense Health Board advised the Joint Trauma System to update the Tactical Combat Casualty Care Guidelines to include ketamine as an acceptable first line agent for pain control on the battlefield. The goal of this study was to investigate trends in the use of ketamine during Operation Enduring Freedom (OEF) and Operation Freedom's Sentinel (OFS) during the years 2011-2016.
Materials and methods:
A retrospective review of Department of Defense Trauma Registry (DoDTR) data was performed for all patients receiving ketamine during OEF/OFS in 2011-2016. Prevalence of ketamine use, absolute use, mechanism of injury, demographics, injury severity score, provider type, and co-administration rates of various medications and blood products were evaluated.
Results:
Total number of administrations during the study period was 866. Ketamine administration during OEF/OFS increased during the years 2011-2013 (28 patient administrations in 2011, 264 administrations in 2012, and 389 administrations in 2013). A decline in absolute use was noted from 2014 to 2016 (98 administrations in 2014, 41 administrations in 2015, and 46 administrations in 2016). The frequency of battlefield ketamine use increased from 0.4% to 11.3% for combat injuries sustained in OEF/OFS from 2011 to 2016. Explosives (51%) and penetrating trauma (39%) were the most common pattern of injury in which ketamine was administered. Ketamine was co-administered with fentanyl (34.4%), morphine (26.2%), midazolam (23.1%), tranexamic acid (12.3%), plasma (10.3%), and packed red blood cells (18.5%).
Extrait du texte:
" Registry data demonstrate that the majority of these administrations were initially documented at role III (785; 82%), and role IIb (164; 17%) facilitie"
Conclusions:
This study demonstrates increasing use of ketamine by the U.S. Military on the battlefield and effectiveness of clinical practice guidelines in influencing practice patterns.
10/04/2021
Ketamine: Le point US
Ketamine Use in Operation Enduring Freedom
Leslie E. et al. Mil Med . 2021 Apr 7;usab117. doi: 10.1093/milmed/usab117
------------------------------------
La Kétamine fait partie maintenant de l'arsenal thérapeutique utilisé par les US. Dans la discussion l'article évoque une probable sous déclaration d'emploi lié à un système de recueil des données encore à nparfaire au niveau des role 1
------------------------------------
Ketamine is a dissociative anesthetic increasingly used in the prehospital and battlefield environment. As an analgesic, it has been shown to have comparable effects to opioids. In 2012, the Defense Health Board advised the Joint Trauma System to update the Tactical Combat Casualty Care Guidelines to include ketamine as an acceptable first line agent for pain control on the battlefield. The goal of this study was to investigate trends in the use of ketamine during Operation Enduring Freedom (OEF) and Operation Freedom’s Sentinel (OFS) during the years 2011-2016.
A retrospective review of Department of Defense Trauma Registry (DoDTR) data was performed for all patients receiving ketamine during OEF/OFS in 2011-2016. Prevalence of ketamine use, absolute use, mechanism of injury, demographics, injury severity score, provider type, and co-administration rates of various medications and blood products were evaluated.
Total number of administrations during the study period was 866. Ketamine administration during OEF/OFS increased during the years 2011-2013 (28 patient administrations in 2011, 264 administrations in 2012, and 389 administrations in 2013). A decline in absolute use was noted from 2014 to 2016 (98 administrations in 2014, 41 administrations in 2015, and 46 administrations in 2016). The frequency of battlefield ketamine use increased from 0.4% to 11.3% for combat injuries sustained in OEF/OFS from 2011 to 2016. Explosives (51%) and penetrating trauma (39%) were the most common pattern of injury in which ketamine was administered. Ketamine was co-administered with fentanyl (34.4%), morphine (26.2%), midazolam (23.1%), tranexamic acid (12.3%), plasma (10.3%), and packed red blood cells (18.5%).
This study demonstrates increasing use of ketamine by the U.S. Military on the battlefield and effectiveness of clinical practice guidelines in influencing practice patterns.
09/02/2021
Analgésie: Pas si évident
Objectives:
The objectives of this study were to assess comparative effectiveness and harms of opioid and nonopioid analgesics for the treatment of moderate to severe acute pain in the prehospital setting.
Methods:
We searched MEDLINE®, Embase®, and Cochrane Central from the earliest date through May 9, 2019. Two investigators screened abstracts, reviewed full-text files, abstracted data, and assessed study level risk of bias. We performed meta-analyses when appropriate. Conclusions were made with consideration of established clinically important differences and we graded each conclusion's strength of evidence (SOE).
Results:
We included 52 randomized controlled trials and 13 observational studies. Due to the absence or insufficiency of prehospital evidence we based conclusions for initial analgesia on indirect evidence from the emergency department setting. As initial analgesics, there is no evidence of a clinically important difference in the change of pain scores with opioids vs. ketamine administered primarily intravenously (IV) (low SOE), IV acetaminophen (APAP) (low SOE), or nonsteroidal anti-inflammatory drugs (NSAIDs) administered primarily IV (moderate SOE). The combined use of an opioid and ketamine, administered primarily IV, may reduce pain more than an opioid alone at 15 and 30 minutes (low SOE). Opioids may cause fewer adverse events than ketamine (low SOE) when primarily administered intranasally. Opioids cause less dizziness than ketamine (low SOE) but may increase the risk of respiratory depression compared with ketamine (low SOE), primarily administered IV. Opioids cause more dizziness (moderate SOE) and may cause more adverse events than APAP (low SOE), both administered IV, but there is no evidence of a clinically important difference in hypotension (low SOE). Opioids may cause more adverse events and more drowsiness than NSAIDs (low SOE), both administered primarily IV.
Conclusions:
As initial analgesia, opioids are no different than ketamine, APAP, and NSAIDs in reducing acute pain in the prehospital setting. Opioids may cause fewer total side effects than ketamine, but more than APAP or NSAIDs. Combining an opioid and ketamine may reduce acute pain more than an opioid alone but comparative harms are uncertain. When initial morphine is inadequate, giving ketamine may provide greater and quicker acute pain relief than giving additional morphine, although comparative harms are uncertain. Due to indirectness, strength of evidence is generally low, and future research in the prehospital setting is needed.
28/11/2020
Kétamine: Recommendations de l'ACEP
| Tags : kétamine
02/02/2020
Penthrox: Pour la sédation procédurale ?
Inhaled methoxyflurane for the reduction of acute anterior shoulder dislocation in the emergency department.
- ------------------------------------------------
OBJECTIVES:
Methoxyflurane is an inhalation analgesic used in the emergency department (ED) but also has minimal sedative properties. The major aim of this study was to evaluate the success rate of methoxyflurane for acute anterior shoulder dislocation (ASD) reduction. The secondary aim was to assess the impact of methoxyflurane on ED patient flow compared to propofol.
METHODS:
A health record review was performed for all patients presenting with ASD who underwent reduction with either methoxyflurane or propofol over a 13-month period (December 2016 - December 2017). The primary outcome was reduction success for methoxyflurane, while secondary outcomes such as recovery time and ED length of stay (LOS) were also assessed compared to propofol. Patients with fracture dislocations, polytrauma, intravenous, or intramuscular opioids in the pre-hospital setting, no sedation for reduction, and alternative techniques of sedation or analgesia for reduction were excluded.
RESULTS:
A total of 151 patients presented with ASD during the study period. Eighty-two patients fulfilled our inclusion criteria. Fifty-two patients had ASD reduction with propofol while 30 patients had methoxyflurane. Successful reduction was achieved in 80% (95% CI 65.69% to 94.31%) patients who used methoxyflurane. The median recovery time and ED LOS were 30 minutes [19.3-44] and 70.5 minutes [49.3-105], which was found to be shorter for the methoxyflurane group, who had successful reductions compared to sedation with propofol.
CONCLUSION:
Methoxyflurane was used successfully in 30% of the 82 patients undergoing reduction for ASD, while potentially improving ED efficiency.
02/01/2020
Methoxyflurane en altitude ? Mais oui
WIlkes M et Al. Wilderness Environ Med. 2018 Sep;29(3):388-391.
Methoxyflurane is a volatile, fluorinated anesthetic agent with analgesic properties. Although no longer used as an anesthetic due to concerns regarding renal toxicity in high doses, it has enjoyed a resurgence as an inhaled analgesic in prehospital care and in the emergency department. The agent is nonflammable and leads to rapid, titratable analgesia without intravenous access. The Penthrox inhaler device is light, robust, and straightforward to administer. Consequently, it has been proposed as an ideal analgesic for the remote high altitude setting.
We report its use for procedural analgesia during suprapubic aspiration for acute urinary retention at a remote rescue post at night, in cold winter conditions, at 4470 m altitude in Machermo, Nepal. We found that methoxyflurane provided rapid, effective analgesia for our patient’s visceral and procedural pain. The inhaler was easy to administer, and the patient remained responsive to voice, with satisfactory oxygen saturation and respiratory rate throughout. We also briefly review the administration, dosing, efficacy, and safety of methoxyflurane and its role in remote medical care.
19/11/2019
Methoxyflurane: Très polluant ?
A Rigorous Evaluation of Methoxyflurane is Needed: Comment on “Methoxyflurane Versus Standard of Care for Acute Trauma-Related Pain in the Emergency Setting: Protocol for a Randomised, Controlled Study in Italy (MEDITA)”
--------------------------------
L'utilisation du Penthrox comme antalgique génère beaucoup de déchets (par ailleurs qui gaz à effets de serre), trop de déchets pour être recommandé? C'est du moins ce que pense une équipe nantaise.
--------------------------------
We have read with careful attention the article, “Methoxyflurane Versus Standard of Care for Acute Trauma-Related Pain in the Emergency Setting: Protocol for a Randomised, Controlled Study in Italy” by Fabbri et al. [1]. The authors present MEDITA (Methoxyflurane in Emergency Department in ITAly), a Phase IIIb, prospective, randomised, active-controlled, parallel-group, open-label, multicentre trial. We agree with the authors that there is a need for better evidence for the use of methoxyflurane in pain management in the emergency department. Low-dose methoxyflurane was approved based on the STOP! trial, which compared methoxyflurane only to placebo in young patients with acute trauma pain [2]. Despite a total absence of trials comparing methoxyflurane to an alternative analgesic available in the emergency department, huge efforts are made to introduce methoxyflurane to European emergency physicians, seen as a “global market” as reported in an online promotional video [3]. Indeed, they consider that methoxyflurane “can fit a very significant market need in terms of getting people out of pain, without them having to take a narcotic on board”.
We noticed that the authors will include patients with moderate pain (Numeric Rating Scale 4–6), and that in these patients, guidelines recommend an oral non opioid analgesic that can be given as soon as in the triage room [4]. Whilst the authors argue that the cost of a morphine treatment is high, we wonder if the authors will include in their analysis the cost and time of preparation and education of the patient for the use of the inhaler, and will compare this to the very cheap and straightforward administration of a pill. A cost-effectiveness analysis does not seem to be planned.
We also question the primary endpoint pain assessment at 10 min. The vast majority of the studies that evaluated pain management in the emergency department had a primary outcome at 30 min, as reported in the evidence-based review from Sin et al. [5]. Thus, the added value of satisfactory analgesia at 10 min versus 30 min is unclear, and may not be seen as clinically relevant. This highly question the long term effect of methoxyflurane and the need for other antalgic treatment after the single dose of methoxyflurane received.
Finally, in the era of global warming, and following the Katowice Climate Change Conference (24th session of the Conference of the Parties), we believe that we cannot promote a treatment that cause such amount of waste, as the hand-held inhaler must be throw out after usage. Emergency physicians must take an active part in protecting our planet and prescribe other more environment-friendly medications for pain management.
For all these reasons, we strongly believe that we need independent trials if we want to avoid another future medical reversal [6]. Two trials have been recently funded by the French ministry of health [7].
| Tags : me
Penthrox: Avis des canadiens
08/10/2019
Bloc ilio-fascial: Docteur ou IDE, Faites en !
Efficacy of Prehospital Analgesia with Fascia Iliaca Compartment Block for Femoral Bone Fractures: A Systematic Review.
INTRODUCTION:
Femoral fractures are painful injuries frequently encountered by prehospital practitioners. Systemic opioids are commonly used to manage the pain after a femoral fracture; however, regional techniques for providing analgesia may provide superior targeted pain relief and reduce opioid requirements. Fascia Iliaca Compartment Block (FICB) has been described as inexpensive and does not require special skills or equipment to perform, giving it the potential to be a suitable prehospital intervention. ProblemThe purpose of this systematic review is to summarize published evidence on the prehospital use of FICB in patients of any age suffering femoral fractures; in particular, to investigate the effects of a prehospital FICB on pain scores and patient satisfaction, and to assess the feasibility and safety of a prehospital FICB, including the success rates, any delays to scene time, and any documented adverse effects.
METHODS:
A literature search of MEDLINE/PubMED, Embase, OVID, Scopus, the Cochrane Database, and Web of Science was conducted from January 1, 1989 through February 1, 2017. In addition, reference lists of review articles were reviewed and the contents pages of the British Journal of Anaesthesia (The Royal College of Anaesthetists [London, UK]; The College of Anaesthetists of Ireland [Dublin, Ireland]; and The Hong Kong College of Anaesthesiologists [Aberdeen, Hong Kong]) 2016 along with the journal Prehospital Emergency Care (National Association of Emergency Medical Service Physicians [Overland Park, Kansas USA]; National Association of State Emergency Medical Service Officials [Falls Church, Virginia USA]; National Association of Emergency Medical Service Educators [Pittsburgh, Pennsylvania USA]; and the National Association of Emergency Medical Technicians [Clinton, Mississippi USA]) 2016 were hand searched. Each study was evaluated for its quality and its validity and was assigned a level of evidence according to the Oxford Centre for Evidence-Based Medicine (OCEBM; Oxford, UK).
RESULTS:
Seven studies involving 699 patients were included (one randomized controlled trial [RCT], four prospective observational studies, one retrospective observational study, and one case report). Pain scores reduced after prehospital FICB across all studies, and some achieved a level of significance to support this. Out of a total of 254 prehospital FICBs, there was a success rate of 90% and only one adverse effect reported.
--------
"Across all studies, a total of 256 FICBs were performed in a prehospital environment by a variety of practitioners (145 by physicians, 100 by nurses, and 11 by paramedics)"
--------
Few studies have investigated the effects of prehospital FICB on patient satisfaction or scene time delays.
CONCLUSIONS AND RELEVANCE:
The FICB is suitable for use in the prehospital environment for the management of femoral fractures. It has few adverse effects and can be performed with a high success rate by practitioners of any background. Studies suggest that FICB is a useful analgesic technique, although further research is required to investigate its effectiveness compared to systemic opioids..
17/09/2019
Penthrox: Ne nous emballons pas
Methoxyflurane in Pre-Hospital Settings: A Review of Clinical Effectiveness, Cost-Effectiveness and Guidelines
--------------------------------
Beaucoup d'intérêt pour cet agent tombé en désuétude en anesthéise du fait d'effets secondaires importants mais utilisé comme antalgique. La HAS a émis un avis pour le moin mitigé. C'est également la position de l'agence du médicament canadienne qui reconnait des effets antalgiques réel mais sans avantage vrai par rapport à la morphine dans le domaine pré-hospitalier.
--------------------------------
The objective of this review is to evaluate the clinical effectiveness, cost-effectiveness, and evidence-based guidelines for the use of low-dose methoxyflurane for the management of moderate to severe pain associated with trauma or procedures in the pre-hospital setting.
29/07/2019
Methoxyflurane en altitude ? Mais oui
WIlkes M et Al. Wilderness Environ Med. 2018 Sep;29(3):388-391.
Methoxyflurane is a volatile, fluorinated anesthetic agent with analgesic properties. Although no longer used as an anesthetic due to concerns regarding renal toxicity in high doses, it has enjoyed a resurgence as an inhaled analgesic in prehospital care and in the emergency department. The agent is nonflammable and leads to rapid, titratable analgesia without intravenous access. The Penthrox inhaler device is light, robust, and straightforward to administer. Consequently, it has been proposed as an ideal analgesic for the remote high altitude setting.

We report its use for procedural analgesia during suprapubic aspiration for acute urinary retention at a remote rescue post at night, in cold winter conditions, at 4470 m altitude in Machermo, Nepal. We found that methoxyflurane provided rapid, effective analgesia for our patient’s visceral and procedural pain. The inhaler was easy to administer, and the patient remained responsive to voice, with satisfactory oxygen saturation and respiratory rate throughout. We also briefly review the administration, dosing, efficacy, and safety of methoxyflurane and its role in remote medical care.
Penthrox: Risque d'hyperthermie maligne ?
An in-vitro model of malignant hyperthermia: differential effects of inhalation anesthetics on caffeine-induced muscle contractures.
Clinical concentrations of anesthetics augment caffeine-induced contracture of frog sartorius muscle; however, anesthetics differ in this characteristic. The potentiation was quantitated using six paired sartorius muscles for each specified concentration of anesthetic and controls. At a concentration of 1 MAC, the greatest potentiation occurred with 2 mM caffeine for all anesthetics studied. Under these conditions the order of magnitude of augmentations was: chloroform (15 times); halothane (11 times); methoxyflurane (10 times); cyclopropane (5 times); enflurane (4 times); isoflurane (3 times); diethyl ether (2 times); Baxter 3224 (2 times); fluroxene (1.4 times); nitrous oxide (1.3 times).
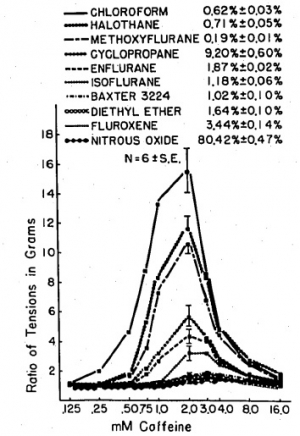
Halothane at .5 MAC augments the 2 mM caffeine-induced contracture almost seven times, and at 2 MAC almost 13 times, whereas 2 MAC isoflurane potentiates the caffeine-induced contracture only four times and 4 MAC diethyl ether only two and a half times. It is postulated that those anesthetics that most potentiate caffeine-induced contracture may be the most potent triggering agents of malignant hyperthermia.
14/07/2019
Penthrox: Séduisant mais
Low-dose methoxyflurane analgesia in adolescent patients with moderate-to-severe trauma pain: a subgroup analysis of the STOP! study.
Introduction:
The undertreatment of acute pain presents a significant challenge in the Emergency Department. This post hoc subgroup analysis of a previously reported randomized controlled UK study reports the efficacy and safety of low-dose methoxyflurane analgesia in treating adolescent patients with moderate-to-severe trauma pain.
Patients and methods:
Three hundred patients (96 in the adolescent subgroup) aged ≥12 years requiring analgesia for acute trauma pain (pain score of 4-7 on the Numerical Rating Scale) at triage were randomized 1:1 to methoxyflurane (up to 6 mL) or placebo (normal saline), both administered using a Penthrox® inhaler. The patient could request rescue medication (paracetamol/opioids) at any time. The primary endpoint was the change from baseline in visual analog scale (VAS) pain intensity.
Results:
Mean VAS pain score for the adolescent subgroup at baseline was ~ 61 mm. Adjusted mean change in VAS pain intensity from baseline to 5, 10, 15, and 20 minutes was -24.5, -28.1, -31.6, and -31.7 mm for methoxyflurane and -14.6, -18.8, -19.2, and -23.7 mm for placebo, with a statistically significant treatment effect in favor of methoxyflurane overall across all four time points (-9.9 mm; 95% CI: -17.4, -2.4 mm; P=0.0104). Median time to first pain relief was significantly shorter with methoxyflurane (1 minute) than placebo (3 minutes, P<0.0001). Pain relief was reported within 1-10 inhalations in 95.7% of methoxyflurane-treated patients and 64.6% of placebo-treated patients. Rescue medication was requested by two (4.3%) methoxyflurane-treated patients and three (6.3%) placebo-treated patients. Over 95% of patients, physicians, and nurses rated methoxyflurane treatment as "Excellent", "Very Good" or "Good" compared with between 64% and 68% for placebo. The incidence of adverse events was higher with methoxyflurane (51%) than placebo (42%), mostly comprising mild/transient dizziness and headache.
Conclusion:
This subgroup analysis shows that low-dose inhaled methoxyflurane is a rapid-acting and effective analgesic in adolescent patients presenting with moderate-to-severe trauma pain.
Penthrox: Avis de la HAS
 Le méthoxyflurane a été utilisé dès 1960 en Europe et aux États-Unis comme gaz anesthésiant halogéné à des doses plus élevées et avec des inhalateurs différents de ceux de PENTHROX. Il a été retiré du marché en 1974 compte tenu d’une faible utilisation en anesthésie. De plus, à fortes doses, le méthoxyflurane a été associé à une néphrotoxicité grave liée à la dose et à l’utilisation pendant de longues périodes au cours d’une anesthésie générale. Sous la dénomination PENTHROX liquide pour inhalation par vapeur en flacon de 3 ml, il est utilisé en Australie depuis 1993 et en Nouvelle Zélande depuis 2002 par les services d’urgence en tant qu’analgésique non opioïde, d’urgence. PENTHROX pourrait être une alternative aux antalgiques comme le MEOPA actuellement utilisés dans ces situations de traumatologie.
Le méthoxyflurane a été utilisé dès 1960 en Europe et aux États-Unis comme gaz anesthésiant halogéné à des doses plus élevées et avec des inhalateurs différents de ceux de PENTHROX. Il a été retiré du marché en 1974 compte tenu d’une faible utilisation en anesthésie. De plus, à fortes doses, le méthoxyflurane a été associé à une néphrotoxicité grave liée à la dose et à l’utilisation pendant de longues périodes au cours d’une anesthésie générale. Sous la dénomination PENTHROX liquide pour inhalation par vapeur en flacon de 3 ml, il est utilisé en Australie depuis 1993 et en Nouvelle Zélande depuis 2002 par les services d’urgence en tant qu’analgésique non opioïde, d’urgence. PENTHROX pourrait être une alternative aux antalgiques comme le MEOPA actuellement utilisés dans ces situations de traumatologie.
La spécialité PENTHROX dispose d’une AMM obtenue par procédure décentralisée dans 3 autres pays de l’union européenne en plus de la France : Belgique, Irlande, Royaume-Uni. Elle dispose d’une AMM en Australie depuis 1993 et en nouvelle Zélande depuis 2002 ; elle est commercialisée depuis ces dates dans ces 2 pays.

PENTHROX est une alternative aux antalgiques disponibles dans la prise en charge en urgence de la douleur modérée à sévère. Faute de données, il n’est pas possible de le situer par rapport aux alternatives antalgiques disponibles. Il présente un avantage pratique (pas de voie veineuse, auto-administration). Son principal inconvénient est la nécessité d’obtenir la coopération du patient, ce qui le contre-indique chez l’adulte inconscient ou au contraire agité. De plus, l’administration par voie inhalée limite son utilisation chez le patient présentant une insuffisance respiratoire chronique ou aiguë.
Les effets indésirables les plus fréquents rapportés avec PENTHROX ont été essentiellement les effets sur le système nerveux central tels que les vertiges, la somnolence et les céphalées. De rares cas d’hépatotoxicité ont été rapportés lors de l’utilisation du méthoxyflurane à dose antalgique.
Compte tenu des données cliniques disponibles versus placebo, PENTHROX apporte une réponse partielle au besoin thérapeutique dans le soulagement de la douleur en situation d’urgence. Il subsiste des incertitudes sur son efficacité dans les douleurs les plus sévères (EN>7). De plus, en l’absence de comparaison directe de qualité méthodologique satisfaisante, il n’est pas possible de le situer par rapport aux alternatives antalgiques disponibles. De ce fait, son impact sur la morbidité et sur la qualité de vie n’est à ce jour pas démontré. Par ailleurs, malgré sa praticité d’utilisation potentielle, son impact positif sur l’organisation des soins n’a pas été démontré.
Le service médical rendu est modéré. Compte tenu de :
– l’efficacité de PENTHROX démontrée versus placebo chez des patients ayant majoritairement une douleur d’intensité modérée,
– l’absence d’étude de qualité méthodologique suffisante l’ayant comparé aux autres antalgiques actuellement disponibles,
PENTHROX n’apporte pas d’amélioration du service médical rendu (ASMR V) par rapport aux autres antalgiques disponibles dans le soulagement d’urgence des douleurs modérées à sévères associées à un traumatisme chez des patients adultes conscients.
16/05/2019
Penthrox: Peut-être MAIS l'hyperthermie maligne ?
Penthrox: a breath of PHEC air for the military?
----------------------------------------
Le penthrox est souvent présenté comme une nouveauté. Il s'agit d'un agent d'anesthésie ancien dont l'emploi a cessé du fait, entre autres, d'une nephrotoxicité importante. Comme tous les agents anesthésiques halogénés, il possède une activité analgésique vraie. Il est aussi potentiellement un agent déclenchant d'hyperthermie maligne anesthésique. Les auteurs font une présentation de ce produit en omettant d'aborder ce problème potentiel dont on sait qu'il touche (délai et doses déclenchantes) de manière diverse tous les halogénés (1)
----------------------------------------
Prehospital analgesia is vital to good clinical care and inhaled methoxyflurane (Penthrox) would be a valuable addition to the armed forces medical armoury. Penthrox would provide strong, fast-acting, self-administered and safe analgesia to patients with moderate to severe injuries. In addition, it would provide an option for strong analgesia which would not be subject to the regulations that govern controlled or accountable drugs which gives it a unique position as the military moves its focus from large enduring operations to small short-term training teams supported by lone combat medics in remote locations across the globe.



