16/12/2025
Quoi d'autre que le sang pour le choc hémorragique: ?
Adjuvant therapies for management of hemorrhagic shock: a narrative review
Daniel Y et Al. Crit Care. 2025 Mar 29;29(1):138
BackgroundSevere bleeding remains a leading cause of death in patients with major trauma, despite improvements in care during the acute phase, especially the application of damage control concepts. Death from hemorrhage occurs rapidly after the initial trauma, in most cases before the patient has had a chance to reach a hospital. Thus, the development of adjuvant drugs that would increase the survival of injured patients is necessary. Among the many avenues of research in this area, one is to improve cell survival during tissue hypoxia. During hemorrhagic shock, oxygen delivery to cells decreases and, despite increased oxygen extraction, anaerobic metabolism occurs, leading to acidosis, coagulopathy, apoptosis, and organ dysfunction.
Methods
We selected six treatments that may help cells cope with this situation and could be used as adjuvant therapies during the initial resuscitation of severe trauma patients, including out-of-hospital settings: niacin, thiazolidinediones, prolyl hydroxylase domain inhibitors, O-GlcNAcylation stimulation, histone deacetylase inhibitors, and adenosine–lidocaine–magnesium solution. For each treatment, the biological mechanism involved and a systematic review of its interest in hemorrhagic shock (preclinical data and human clinical trials) are presented.

Conclusion
Promising molecules, some of which are already used in humans for other indications, give us hope for human clinical trials in the field of hemorrhagic shock in the near future
13/12/2025
Ketamine intranasale: Bon pour le service ?
Breathing new life into pain management: a systematic review of nebulised ketamine for analgesia
Daniel Kirk D et Al. Scand J Trauma Resusc Emerg Med. 2025 Dec 4;33(1):196.
Background:
Acute pain accounts for 60-90% of presentations to the emergency department (ED), with 20-40% of patients reporting severe pain. Current management practices, including simple analgesics, opiates and anti-inflammatory drugs, are often inadequate or slow to reach peak effect, necessitating the exploration of alternative analgesics. Ketamine, acting primarily through N-methyl-D-aspartate (NMDA) receptor antagonism, presents a promising alternative due to its rapid onset. However, its nebulised form remains underutilised in clinical practice.
Aims and objectives:
This review evaluates the efficacy of nebulised ketamine in reducing pain in adult ED patients, alongside its side effect profile, optimal dosing, and potential as an alternative or adjunctive analgesic compared to other treatments.
Methods and design:
A systematic review utilising the PRISMA guidelines was conducted. Searches were carried out in Medline, Embase, PubMed, Science Direct, google scholar and Ovid databases from 2010 to May 2024, including studies containing objective analysis of pain control with nebulised ketamine. A two-sample t-test was used to assess statistical significance. Quality assessment was performed using the CASP tool, and bias was evaluated using the ROBINS-I and ROB2 tool.
Results:
Of 99 articles, 9 (5 randomised controlled trials, 3 case series and 1 case report) totalling 453 patients were included. All studies suggested improvement in pain scores with nebulised ketamine, with an average reduction of 42.5% and 70.4% over a 15 and 120-minute period respectively (p < 0.0001). Higher doses (1 mg/kg, 1.5 mg/kg) did not significantly improve pain compared to lower doses (0.7 mg/kg 0.75 mg/kg), with similar overall reductions reported across all four dosing regimens (p < 0.0003 or 0.0001).
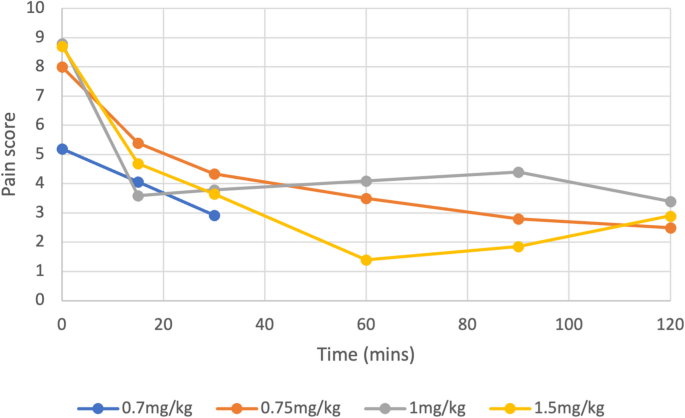
Nebulised ketamine was non-inferior to intravenous (IV) morphine, IV ketamine, nebulised dexmedetomidine, and Entonox, and had fewer side effects.
Conclusion: Nebulised ketamine offers a viable alternative for pain management in emergency settings, providing effective analgesia with a favourable safety profile. Further multicentre trials with larger populations are recommended to confirm these findings and establish standardised dosing protocols for consideration in national guidance.
12/12/2025
TXA: 2g IV si trauma crânien
Optimal dose of tranexamic acid in traumatic brain injury: Systematic review and network meta-analysis of randomized controlled trials
Shu U et Al Journal of Trauma and Acute Care Surgery 98(5):p 816-823, May 2025.
BACKGROUND
Tranexamic acid (TXA) has been used to treat traumatic brain injury (TBI); however, no definitive conclusions have been drawn regarding its effectiveness or dosage. This study evaluated the optimal TXA dose for treating TBI using a network meta-analysis (NMA).
METHODS
Five databases were searched for peer-reviewed randomized controlled trials (RCTs) published from inception to May 2024. The inclusion criteria were as follows: (1) RCTs, (2) patients older than 1 month with TBI, (3) interventions of TXA and control, (4) primary outcomes of mortality and poor neurological outcomes and secondary outcomes of vascular occlusive events, and (5) full-text peer-reviewed articles. Two reviewers independently screened and extracted the data and assessed the risk of bias. Frequency-based NMA was performed using the Grading of Recommendations, Assessment, Development, and Evaluation working-group approach.
RESULTS
We included 10 RCTs comprising 11,237 patients with TBI. Placebo showed higher mortality compared with that of a 2-g bolus of TXA (risk ratio, 1.53; 95% confidence interval, 1.08–2.17). Higher mortality was observed with a 1-g bolus of TXA followed by 1-g maintenance TXA compared with that of a 2-g bolus of TXA (risk ratio, 1.44; 95% confidence interval, 1.02–2.03). No significant differences in poor neurological outcomes or vascular occlusive events were observed between the treatment groups.

CONCLUSION
Placebo and a 1-g bolus followed by 1-g maintenance TXA were associated with higher mortality rates than those of a 2-g bolus of TXA. No difference in vascular occlusive events was observed with either treatment, indicating that our NMA recommends 2 g of TXA. However, the data for the 2-g bolus of TXA were from a single study, and further research is needed to draw definitive conclusions.
07/12/2025
Vers un nouveau Plyo
Efficacy and safety of novel freeze-dried plasma products in a porcine combat casualty model
Dufour-Gaume F et Al. Transfusion. 2024 Sep;64(9):1670-1682
-----------
Le Plyo est un des produits phares développé par le CTSA. Les recherches continuent pour améliorer les caractérisques. C'est le sens de ce tavail qui porte sur du Plyo plus concentré ou enrichi en lyophylisat de plaquettes. Ce dernier produit parait particulièrement intéressant. l'industrie pharmaceutique n'est pas en reste puisqu'elle propose maintenant des alternatives au PLyo standard comme l'OctaplastLG considéré comme un médicament et non plus un dérivé du sang.
-----------
Background
Hemorrhagic shock is well documented as a leading cause of preventable fatalities among military casualties. During military operations plasma can be transfused while waiting for whole blood. This study was conducted to assess the safety and efficacy of two new freeze-dried plasma formulations in a porcine model of traumatic hemorrhagic shock.
Study Design and Methods
In the face of species-specific transfusion, transfusible blood products were derived from porcine sources. The efficacy of three lyophilized plasma (LP) formulations was evaluated: lyophilized plasma (LP), concentrated lyophilized plasma (CLP), and platelet-rich concentrated lyophilized plasma (PCLP). Pigs were subjected to multi-trauma and hemorrhagic shock. Ninety minutes post-shock induction, the animals were treated with one of the three lyophilized products. Monitoring included systolic blood pressure and cardiac output. Point-of-care and laboratory diagnostic tests were used to assess renal function, real-time hemostasis (ROTEM), and coagulation. Histological examinations of kidney, lung, and muscle tissues were conducted 4 h after shock induction.
Results
CLP and PCLP significantly improved systolic blood pressure and cardiac output and positively influenced base excess, creatinine, various ROTEM, and coagulation markers compared with standard LP without histologic modification. No adverse effect was associated with the transfusion of any of the plasma products throughout the experimental procedures.
Conclusion
Both CLP and PCLP exhibit promising therapeutic potential for managing hemorrhagic shock in scenario where whole blood supplies are limited. However, the distinct physiological and coagulation characteristics of the swine model necessitate further investigation using humanized preclinical models to fully understand their clinical applicability and constraints
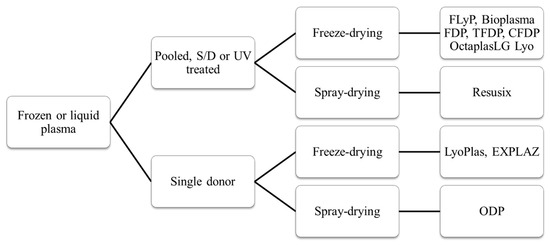
Plasma: Un point en 2024
Dried Plasma for Major Trauma: Past, Present, and Future
05/12/2025
Point US sur l'emploi du TXA
Tranexamic acid in trauma: A joint position statement and resource document of NAEMSP, ACEP, and ACS-COT
Prehospital use of tranexamic acid (TXA) has grown substantially over the past decade despite contradictory evidence supporting its widespread use. Since the previous guidance document on the prehospital use of TXA for injured patients was published by the National Association of EMS Physicians, the American College of Surgeons Committee on Trauma, and the American College of Emergency Physicians in 2016, new research has investigated outcomes of patients who receive TXA in the prehospital setting. To provide updated evidence-based guidance on the use of intravenous TXA for injured patients in the emergency medical services (EMS) setting, we performed a structured literature review and developed the following recommendations supported by the evidence summarized in the accompanying resource document.
The National Association of EMS Physicians, the American College of Surgeons Committee on Trauma, and the American College of Emergency Physicians recommends:
• Prehospital TXA administration may reduce mortality in adult trauma patients with hemorrhagic shock when administered after lifesaving interventions.
• Prehospital TXA administration appears safe, with low risk of thromboembolic events or seizure.
• The ideal dose, rate, and route of prehospital administration of TXA for adult trauma patients with hemorrhagic shock has not been determined. Current evidence suggests EMS agencies may administer either a 1-g intravenous/intraosseous dose (followed by a hospital-based 1-g infusion over 8 hours) or a 2-g intravenous/intraosseous dose as an infusion or slow push.
• Prehospital TXA administration, if used for adult trauma patients, should be given to those with clinical signs of hemorrhagic shock and no later than 3 hours post-injury. There is no evidence to date to suggest improved clinical outcomes from TXA initiation beyond this time or in those without clinically significant bleeding.
• The role of prehospital TXA in pediatric trauma patients with clinical signs of hemorrhagic shock has not been studied, and standardized dosing has not been established. If used, it should be given within 3 hours of injury.
• Prehospital TXA administration, if used, should be clearly communicated to receiving health care professionals to promote appropriate monitoring and to avoid duplicate administration(s).
• A multidisciplinary team, led by EMS physicians, that includes EMS clinicians, emergency physicians, and trauma surgeons should be responsible for developing a quality improvement program to assess prehospital TXA administration for protocol compliance and identification of clinical complications.
12/11/2024
PLyo: Oui mais sans hémodilution par les crstalloïdes
Prehospital Lyophilized Plasma Transfusion for Trauma-Induced Coagulopathy in Patients at Risk for Hemorrhagic Shock. A Randomized Clinical Trial
Jost D. et Al.JAMA Netw Open. 2022;5(7):e2223619.
-----------------
Le recours au plasma fait partie de l'arsenal thérapeutique de la prise en charge des traumatisés sévères en choc hémorragique. Comme l'étude REPHILL (1), Ce travail ne met pas en évidence d'amélioration de la survie à 28 jours. Les délais de transport et le degré d'hémodilution lié au recours aux solutés salés expliquent peut être cela (2)
Key Points
Question Does prehospital transfusion of lyophilized plasma result in a lower incidence of trauma-induced coagulopathy at hospital admission compared with standard care with normal saline infusion in patients at risk for hemorrhagic shock after trauma?
Findings This multicenter randomized clinical trial included 150 patients with trauma who were treated in a prehospital setting. Median international normalized ratio at hospital admission, massive transfusion rate, 30-day survival, and adverse events did not significantly differ between lyophilized plasma transfusion and standard care.
Meaning These findings suggest that prehospital lyophilized plasma transfusion is a feasible and safe procedure for patients who are at risk for hemorrhagic shock, although there is a lack of evidence regarding its ability to prevent trauma-induced coagulopathy.
Abstract
Importance Blood transfusion is a mainstay of therapy for trauma-induced coagulopathy, but the optimal modalities for plasma transfusion in the prehospital setting remain to be defined.
Objective To determine whether lyophilized plasma transfusion can reduce the incidence of trauma-induced coagulopathy compared with standard care consisting of normal saline infusion.
Design, Setting, and Participants This randomized clinical trial was performed at multiple centers in France involving prehospital medical teams. Participants included 150 adults with trauma who were at risk for hemorrhagic shock and associated coagulopathy between April 1, 2016, and September 30, 2019, with a 28-day follow-up. Data were analyzed from November 1, 2019, to July 1, 2020.
Intervention Patients were randomized in a 1:1 ratio to receive either plasma or standard care with normal saline infusion (control).
Main Outcomes and Measures The primary outcome was the international normalized ratio (INR) on arrival at the hospital. Secondary outcomes included the need for massive transfusion and 30-day survival. As a safety outcome, prespecified adverse events included thrombosis, transfusion-related acute lung injury, and transfusion-associated circulatory overload.
Results Among 150 randomized patients, 134 were included in the analysis (median age, 34 [IQR, 26-49] years; 110 men [82.1%]), with 68 in the plasma group and 66 in the control group. Median INR values were 1.21 (IQR, 1.12-1.49) in the plasma group and 1.20 (IQR, 1.10-1.39) in the control group (median difference, −0.01 [IQR, −0.09 to 0.08]; P = .88). The groups did not differ significantly in the need for massive transfusion (7 [10.3%] vs 4 [6.1%]; relative risk, 1.78 [95% CI, 0.42-8.68]; P = .37) or 30-day survival (hazard ratio for death, 1.07 [95% CI, 0.44-2.61]; P = .89). In the full intention-to-treat population (n = 150), the groups did not differ in the rates of any of the prespecified adverse events.
Conclusions and Relevance In this randomized clinical trial including severely injured patients at risk for hemorrhagic shock and associated coagulopathy, prehospital transfusion of lyophilized plasma was not associated with significant differences in INR values vs standard care with normal saline infusion. Nevertheless, these findings show that lyophilized plasma transfusion is a feasible and safe procedure for this patient population.
09/10/2024
Analgésie par inhalation ? Pas un plébiscite
Inhaled analgesics for the treatment of prehospital acute pain—A systematic review
Hyldmo PK et Al. Acta Anaesthesiol Scand. 2024 Sep 26. doi: 10.1111/aas.1452
Background
Many prehospital emergency patients receive suboptimal treatment for their moderate to severe pain. Various factors may contribute. We aim to systematically review literature pertaining to prehospital emergency adult patients with acute pain and the pain-reducing effects, adverse events (AEs), and safety issues associated with inhaled analgetic agents compared with other prehospital analgesic agents.
Methods
As part of an initiative from the Scandinavian Society of Anaesthesia and Intensive Care Medicine, we conducted a systematic review (PROSPERO CRD42018114399), applying the PRISMA guidelines, Grading of Recommendations Assessment, Development, and Evaluation (GRADE), and Cochrane methods, searching the Cochrane Library, Epistemonikos, Centre for Reviews and Dissemination, PubMed, and EMBASE databases (updated March 2024). Inclusion criteria were the use of inhaled analgesic agents in adult patients with acute pain in the prehospital emergency care setting. All steps were performed by minimum of two individual researchers. The primary outcome was pain reduction; secondary outcomes were speed of onset, duration of effect, and relevant AEs.
Results
We included seven studies (56,535 patients in total) that compared inhaled agents (methoxyflurane [MF] and nitrous oxide [N2O]) to other drugs or placebo. Study designs were randomized controlled trial (1; n = 60), randomized non-blinded study (1; n = 343), and randomized open-label study (1; n = 270). The remaining were prospective or retrospective observational studies. The evidence according to GRADE was of low or very low quality. No combined meta-analysis was possible. N2O may reduce pain compared to placebo, but not compared to intravenous (IV) paracetamol, and may be less effective compared to morphine and MF. MF may reduce pain compared to paracetamol, ketoprofen, tramadol, and fentanyl. Both agents may be associated with marked but primarily mild AEs.
Conclusion
We found low-quality evidence suggesting that both MF and N2O are safe and may have a role in the management of pain in the prehospital setting. There is low-quality evidence to support MF as a short-acting single analgesic or as a bridge to IV access and the administration of other analgesics. There may be occupational health issues regarding the prehospital use of N2O.
24/09/2024
Plasma; Et l'OctaplasLG ?
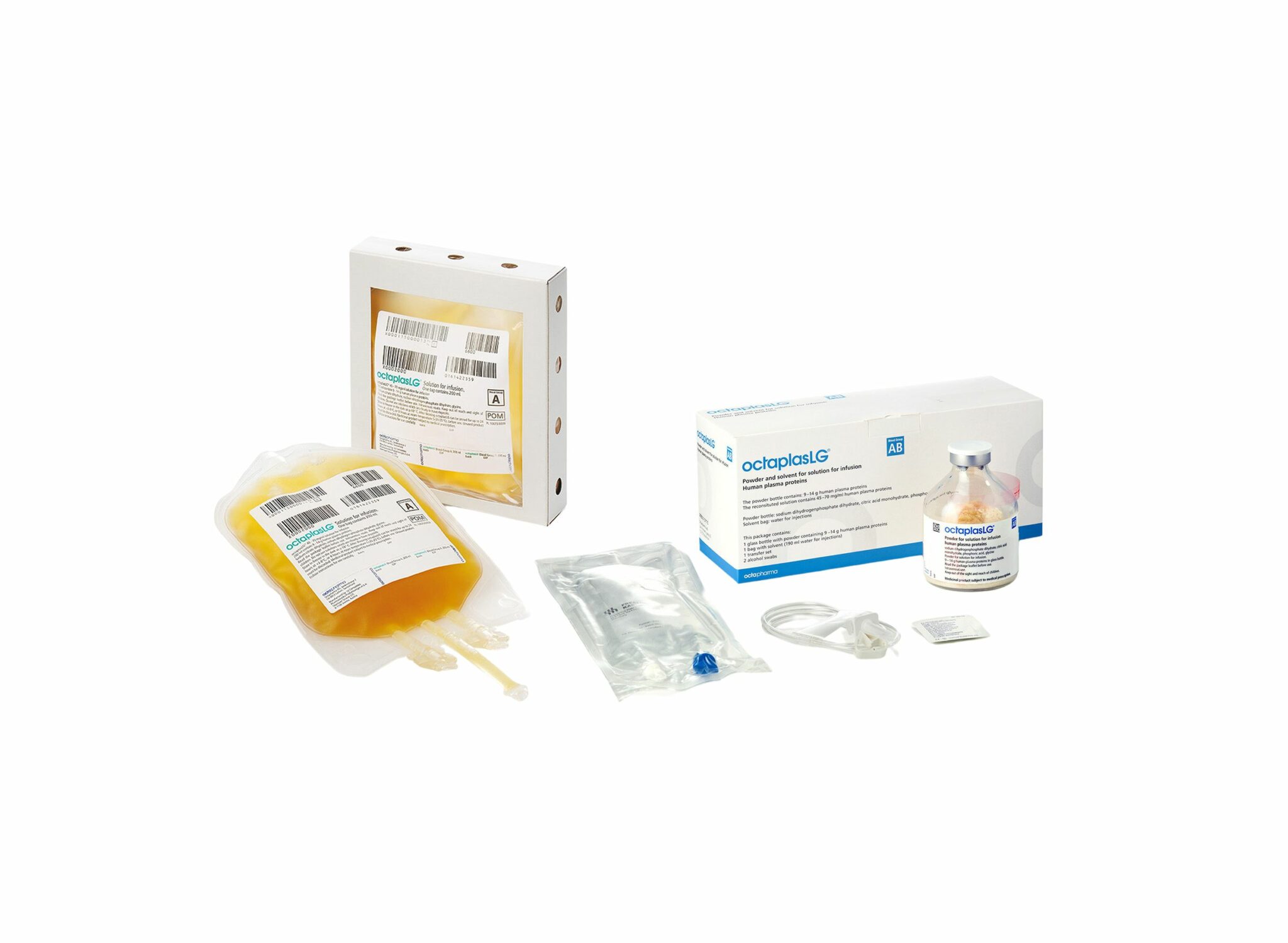
Une alternative HOSPITALIERE au PLyo du fait d'une production actuelle limitée ? VIent d'obtenir son agréement en Europe. Plus simple en gestion car considéré comme un médicament et non un produit sanguin. Il est fourni en présentations distinctes, selon les groupes sanguins. L’administration d’OCTAPLASLG doit respecter les règles de compatibilité des groupes sanguins ABO. En cas d’urgence, OCTAPLASLG du groupe AB peut être considéré comme plasma universel dans la mesure où il peut être administré à tous les patients, indépendamment de leur groupe sanguin. C'est donc dans cette forme qu'il peut être utilisé en préhospitalier comme le Plyo.
Sa place reste à préciser
03/04/2023
Sang de banque sur pied: Mieux si on est entraîné
A prospective assessment of the medic autologous blood transfusion skills for field transfusion preparation
Steven G Schauer SG et Al. Transfusion. 2023 Mar 27. doi: 10.1111/trf.17325.
Background: Data demonstrate benefit from blood product administration near point-of-injury (POI). Fresh whole blood transfusion from a pre-screened donor provides a source of blood at the POI when resources are constrained. We captured transfusion skills data for medics performing autologous blood transfusion training.
Methods: We conducted a prospective, observational study of medics with varying levels of experience. Inexperienced medics were those with minimal or no reported experience learning the autologous transfusion procedures, versus reported experience among special operations medics. When available, medics were debriefed after the procedure for qualitative feedback. We followed them up to 7 days for adverse events.
Results: The median number of attempts for inexperienced and experienced medics was 1 versus 1 (interquartile range 1-1 for both, p=0.260). The inexperienced medics had a slower median time to needle venipuncture access for donation of 7.3 versus 1.5 minutes, needle removal after clamping time of 0.3 versus 0.2 minutes, time to bag preparation of 1.9 versus 1.0 minutes, time to IV access for reinfusion of 6.0 versus 3.0 minutes, time to transfusion completion of 17.3 versus 11.0 minutes, and time to IV removal of 0.9 versus 0.3 minutes (all p<0.05). We noted one administrative safety event in which allogeneic transfusion occurred. No major adverse events occurred. Qualitative data saturated around the need for quarterly training.
Conclusions: Inexperienced medics have longer procedure times when training autologous whole blood transfusion skills. This data will help establish training measures of performance for skills optimization when learning this procedure.
25/11/2022
Point sur les matériaux hémostatiques
Emerging hemostatic materials for non-compressible hemorrhage control
Dong R. et Al. ,Natl Sci Rev. 2022 Aug 17;9(11):nwac162.
Non-compressible hemorrhage control is a big challenge in both civilian life and the battlefield, causing a majority of deaths among all traumatic injury mortalities. Unexpected non-compressible bleeding not only happens in pre-hospital situations but also leads to a high risk of death during surgical processes throughout in-hospital treatment. Hemostatic materials for pre-hospital treatment or surgical procedures for non-compressible hemorrhage control have drawn more and more attention in recent years and several commercialized products have been developed. However, these products have all shown non-negligible limitations and researchers are focusing on developing more effective hemostatic materials for non-compressible hemorrhage control. Different hemostatic strategies (physical, chemical and biological) have been proposed and different forms (sponges/foams, sealants/adhesives, microparticles/powders and platelet mimics) of hemostatic materials have been developed based on these strategies. A summary of the requirements, state-of-the-art studies and commercial products of non-compressible hemorrhage-control materials is provided in this review with particular attention on the advantages and limitations of their emerging forms, to give a clear understanding of the progress that has been made in this area and the promising directions for future generations.
14/02/2022
Kétamine: Stable longtemps quand il fait très chaud
Ketamine Stability over Six Months of Exposure to Moderate and High Temperature Environments
Foertsch MJ et Al. Prehosp Emerg Care. 2021 Jun 21;1-6.
Background:
All medications should be stored within temperature ranges defined by manufacturers, but logistical and operational challenges of prehospital and military settings complicate adherence to these recommendations. Lorazepam and succinylcholine experience clinically relevant heat-related degradation, whereas midazolam does not. Because ketamine's stability when stored outside manufacturer recommendations is unknown, we evaluated the heat-related degradation of ketamine exposed to several temperature ranges.
Methods: One hundred twenty vials of ketamine (50 mg/mL labeled concentration) from the same manufacturer lot were equally distributed and stored for six months in five environments: an active EMS unit in southwest Ohio (May-October 2019); heat chamber at constant 120 °F (C1); heat chamber fluctuating over 24 hours from 86 °F-120 °F (C2); heat chamber fluctuating over 24 hours from 40 °F-120 °F (C3); heat chamber kept at constant 70 °F (manufacturer recommended room temperature, C4). Four ketamine vials were removed every 30 days from each environment and sent to an FDA-accredited commercial lab for high performance liquid chromatography testing. Data loggers and thermistors allowed temperature recording every minute for all environments. Cumulative heat exposure was quantified by mean kinetic temperature (MKT), which accounts for additional heat-stress over time caused by temperature fluctuations and is a superior measure than simple ambient temperature. MKT was calculated for each environment at the time of ketamine removal. Descriptive statistics were used to describe the concentration changes at each time point.
Results: The MKT ranged from 73.6 °F-80.7 °F in the active EMS unit and stayed constant for each chamber (C1 MKT: 120 °F, C2 MKT: 107.3 °F, C3 MKT: 96.5 °F, C4 MKT: 70 °F). No significant absolute ketamine degradation, or trends in degradation, occurred in any environment at any time point. The lowest median concentration occurred in the EMS-stored samples removed after 6 months [48.2 mg/mL (47.75, 48.35)], or 96.4% relative strength to labeled concentration.
Conclusion: Ketamine samples exhibited limited degradation after 6 months of exposure to real world and simulated extreme high temperature environments exceeding manufacturer recommendations. Future studies are necessary to evaluate ketamine stability beyond 6 months.
| Tags : kétamine
09/02/2022
Efficacité et sécurité de la kétamine pour l'analgésie préhospitalière du blessé de guerre
09/07/2021
L'oxygène: Contrainte logistique à ne pas oublier pour demain
Oxygen Management During Collective Aeromedical Evacuation of 36 COVID-19 Patients With ARDS
Beaussac M. et Al. Mil Med. 2021 Jul 1;186(7-8):e667-e671.
Objective:
The ongoing coronavirus disease-2019 pandemic leads to the saturation of critical care facilities worldwide. Collective aeromedical evacuations (MEDEVACS) might help rebalance the demand and supply of health care. If interhospital transport of patients suffering from ARDS is relatively common, little is known about the specific challenges of collective medevac. Oxygen management in such context is crucial. We describe our experience with a focus on this resource.
Methods:
We retrospectively analyzed the first six collective medevac performed during the coronavirus disease-2019 pandemic by the French Military Health Service from March 17 to April 3, 2020. Oxygen management was compliant with international guidelines as well as aeronautical constraints and monitored throughout the flights. Presumed high O2 consumers were scheduled to board the last and disembark the first.
Results:
Thirty-six mechanically ventilated patients were successfully transported within Europe. The duration of onboard ventilation was 185 minutes (145-198.5 minutes), including the flight, the boarding and disembarking periods. Oxygen intake was 1,650 L per patient per flight (1,350-1,950 L patient per flight) and 564 L per patient per hour (482-675 L per patient-1 per hour) and surpassed our anticipation. As anticipated, presumed high O2 consumers had a reduced ventilation duration onboard. The estimations of oxygen consumptions were frequently overshot, and only two hypoxemia episodes occurred.
Conclusion:
Oxygen consumption was higher than expected, despite anticipation and predefined oxygen management measures, and encourages to a great caution in the processing of such collective medevac missions.
Kétamine: Pas assez utilisée en role 1 ?
Ketamine Use in Operation Enduring Freedom
Eric L. et Al. Mil Med. 2021 Jul 1;186(7-8):e720-e725
Introduction:
Ketamine is a dissociative anesthetic increasingly used in the prehospital and battlefield environment. As an analgesic, it has been shown to have comparable effects to opioids. In 2012, the Defense Health Board advised the Joint Trauma System to update the Tactical Combat Casualty Care Guidelines to include ketamine as an acceptable first line agent for pain control on the battlefield. The goal of this study was to investigate trends in the use of ketamine during Operation Enduring Freedom (OEF) and Operation Freedom's Sentinel (OFS) during the years 2011-2016.
Materials and methods:
A retrospective review of Department of Defense Trauma Registry (DoDTR) data was performed for all patients receiving ketamine during OEF/OFS in 2011-2016. Prevalence of ketamine use, absolute use, mechanism of injury, demographics, injury severity score, provider type, and co-administration rates of various medications and blood products were evaluated.
Results:
Total number of administrations during the study period was 866. Ketamine administration during OEF/OFS increased during the years 2011-2013 (28 patient administrations in 2011, 264 administrations in 2012, and 389 administrations in 2013). A decline in absolute use was noted from 2014 to 2016 (98 administrations in 2014, 41 administrations in 2015, and 46 administrations in 2016). The frequency of battlefield ketamine use increased from 0.4% to 11.3% for combat injuries sustained in OEF/OFS from 2011 to 2016. Explosives (51%) and penetrating trauma (39%) were the most common pattern of injury in which ketamine was administered. Ketamine was co-administered with fentanyl (34.4%), morphine (26.2%), midazolam (23.1%), tranexamic acid (12.3%), plasma (10.3%), and packed red blood cells (18.5%).
Extrait du texte:
" Registry data demonstrate that the majority of these administrations were initially documented at role III (785; 82%), and role IIb (164; 17%) facilitie"
Conclusions:
This study demonstrates increasing use of ketamine by the U.S. Military on the battlefield and effectiveness of clinical practice guidelines in influencing practice patterns.
22/06/2021
RSI: Que fait on ?
Rapid sequence induction: An international survey
Klucka J et Al. Eur J Anaesthesiol. 2020 Jun;37(6):435-442
-----------------------------------
La kétamine, la kétamine la kétamine, la kéta......, la............ Mais pour vous ouvrir l'esprit lisez donc également cet article
-----------------------------------
Background: Rapid sequence induction (RSI) is a standard procedure, which should be implemented in all patients with a risk of aspiration/regurgitation during anaesthesia induction.
Objective: The primary aim was to evaluate clinical practice in RSI, both in adult and paediatric populations.
Design: Online survey.
Settings: A total of 56 countries.
Participants: Members of the European Society of Anaesthesiology.
Main outcome measures: The aim was to identify and describe the actual clinical practice of RSI related to general anaesthesia.
Results: From the 1921 respondents, 76.5% (n=1469) were qualified anaesthesiologists. When anaesthetising adults, the majority (61.7%, n=1081) of the respondents preoxygenated patients with 100% O2 for 3 min and 65.9% (n=1155) administered opioids during RSI.
The Sellick manoeuvre was used by 38.5% (n=675) and was not used by 37.4% (n=656) of respondents. First-line medications for a haemodynamically stable adult patient were propofol (90.6%, n=1571) and suxamethonium (56.0%, n=932). Manual ventilation (inspiratory pressure <12 cmH2O) was used in 35.5% (n=622) of respondents. In the majority of paediatric patients, 3 min of preoxygenation (56.6%, n=817) and opioids (54.9%, n=797) were administered. The Sellick manoeuvre and manual ventilation (inspiratory pressure <12 cmH2O) in children were used by 23.5% (n=340) and 35.9% (n=517) of respondents, respectively. First-line induction drugs for a haemodynamically stable child were propofol (82.8%, n=1153) and rocuronium (54.7%, n=741).
Conclusion: We found significant heterogeneity in the daily clinical practice of RSI. For patient safety, our findings emphasise the need for international RSI guidelines
| Tags : intubation, kétamine
10/04/2021
Ketamine: Le point US
Ketamine Use in Operation Enduring Freedom
Leslie E. et al. Mil Med . 2021 Apr 7;usab117. doi: 10.1093/milmed/usab117
------------------------------------
La Kétamine fait partie maintenant de l'arsenal thérapeutique utilisé par les US. Dans la discussion l'article évoque une probable sous déclaration d'emploi lié à un système de recueil des données encore à nparfaire au niveau des role 1
------------------------------------
Ketamine is a dissociative anesthetic increasingly used in the prehospital and battlefield environment. As an analgesic, it has been shown to have comparable effects to opioids. In 2012, the Defense Health Board advised the Joint Trauma System to update the Tactical Combat Casualty Care Guidelines to include ketamine as an acceptable first line agent for pain control on the battlefield. The goal of this study was to investigate trends in the use of ketamine during Operation Enduring Freedom (OEF) and Operation Freedom’s Sentinel (OFS) during the years 2011-2016.
A retrospective review of Department of Defense Trauma Registry (DoDTR) data was performed for all patients receiving ketamine during OEF/OFS in 2011-2016. Prevalence of ketamine use, absolute use, mechanism of injury, demographics, injury severity score, provider type, and co-administration rates of various medications and blood products were evaluated.
Total number of administrations during the study period was 866. Ketamine administration during OEF/OFS increased during the years 2011-2013 (28 patient administrations in 2011, 264 administrations in 2012, and 389 administrations in 2013). A decline in absolute use was noted from 2014 to 2016 (98 administrations in 2014, 41 administrations in 2015, and 46 administrations in 2016). The frequency of battlefield ketamine use increased from 0.4% to 11.3% for combat injuries sustained in OEF/OFS from 2011 to 2016. Explosives (51%) and penetrating trauma (39%) were the most common pattern of injury in which ketamine was administered. Ketamine was co-administered with fentanyl (34.4%), morphine (26.2%), midazolam (23.1%), tranexamic acid (12.3%), plasma (10.3%), and packed red blood cells (18.5%).
This study demonstrates increasing use of ketamine by the U.S. Military on the battlefield and effectiveness of clinical practice guidelines in influencing practice patterns.
14/03/2021
Hydrazine: Qu'est ce que c'est ?
The Toxicity, Pathophysiology, and Treatment of Acute Hydrazine Propellant Exposure: A Systematic Review
Nguyen HN et Al. Mil Med. 2021 Feb 26;186(3-4):e319-e326.
-----------------------------------
L'hydrazine est employé comme combustible dans les fusées et dans les F16 américains en tant que combustible alimentant une unité de puissance de secours. Et cela n'est pas sans conséquence lors d'une intervention auprès d'un tel type d'aéronef.
-----------------------------------
Introduction:
Hydrazines are highly toxic inorganic liquids that are used as propellants in military and aviation industries, such as the U.S. Air Force F-16 Emergency Power Unit and SpaceX SuperDraco Rockets. The most commonly used derivatives include hydrazine, monomethylhydrazine, and 1,1-dimethylhydrazine (unsymmetrical dimethylhydrazine). Industrial workers in close contact with hydrazines during routine maintenance tasks can be exposed to levels well above the National Institute for Occupational Safety and Health relative exposure limits.
Materials and methods: A systematic review was performed using PubMed, Web of Science, Google Scholar, National Aeronautics and Space Administration Technical Server, and Defense Technical Information Center, and data related to hydrazine exposures were searched from inception to April 2020. Publications or reports addressing hydrazine toxicity, pathophysiology, and treatment of hydrazine fuel exposure were selected.
Results: Acute toxic exposures to hydrazine and its derivatives are rare. There are few case reports of acute toxic exposure in humans, and data are largely based on animal studies. The initial search identified 741 articles, manuscripts, and government reports. After screening for eligibility, 51 were included in this review. Eight articles reported acute exposures to hydrazine propellant in humans, and an additional 14 articles reported relevant animal data.
Conclusions: Exposure to small amounts of hydrazine and its derivatives can cause significant soft tissue injury, pulmonary injury, seizures, coma, and death. Neurologic presentations can vary based on exposure compound and dose. Decontamination is critical as treatment is mainly supportive. High-dose intravenous pyridoxine has been suggested as treatment for hydrazine-related neurologic toxicity, but this recommendation is based on limited human data. Despite recent research efforts to generate less toxic alternatives to hydrazine fuel, it will likely continue to have a role in military and aviation industries. Aerospace and military physicians should be aware of the toxicity associated with hydrazine exposure and be prepared to treat hydrazine toxicity in at-risk populations.
02/02/2021
TXA: Oui, mais parfois pas nécessaire
Unjustified Administration in Liberal Use of Tranexamic Acid in Trauma Resuscitation
Tareq Kheirbek T et Al. J Surg Res . 2021 Feb;258:125-131. doi: 10.1016/j.jss.2020.08.045.
Background:
Early administration of tranexamic acid (TXA) has been widely implemented for the treatment of presumed hyperfibrinolysis in hemorrhagic shock. We aimed to characterize the liberal use of TXA and whether unjustified administration was associated with increased venous thrombotic events (VTEs).
Methods:
We identified injured patients who received TXA between January 2016 and January 2018 by querying our Level 1 trauma center's registry. We retrospectively reviewed medical records and radiologic images to classify whether patients had a hemorrhagic injury that would have benefited from TXA (justified) or not (unjustified).
Results:
Ninety-five patients received TXA for traumatic injuries, 42.1% were given by emergency medical services. TXA was considered unjustified in 35.8% of the patients retrospectively and in 52% of the patients when given by emergency medical services. Compared with unjustified administration, patients in the justified group were younger (47.6 versus 58.4; P = 0.02), more hypotensive in the field (systolic blood pressure: 107 ± 31 versus 137 ± 32 mm Hg; P < 0.001) and in the emergency department (systolic blood pressure: 97 ± 27 versus 128 ± 27; P < 0.001), and more tachycardic in emergency department (heart rate: 99 ± 29 versus 88 ± 19; P = 0.04). The justified group also had higher injury severity score (median 24 versus 11; P < 0.001), was transfused more often (81.7% versus 20.6%; P < 0.001), and had higher in-hospital mortality (39.3% versus 2.9%; P < 0.001), but there was no difference in the rate of VTE (8.2% versus 5.9%).
Conclusions:
Our results highlight a high rate of unjustified administration, especially in the prehospital setting. Hypotension and tachycardia were indications of correct use. Although we did not observe a difference in VTE rates between the groups, though, our study was underpowered to detect a difference. Cautious implementation of TXA in resuscitation protocols is encouraged in the meantime. Nonetheless, adverse events associated with unjustified TXA administration should be further evaluated
08/12/2020
Lidocaïne IV: Cette oubliée
The use of intravenous lidocaine for postoperative pain and recovery: international consensus statement on efficacy and safety
Foo I. et Al. Anaesthesia . 2020 Nov 3. doi: 10.1111/anae.15270.
Clic sur l'image pour accéder au document