14/09/2016
X stat: Magique ? Et bien NON
Management of External Hemorrhage in Tactical Combat Casualty Care: The Adjunctive Use of XStat™ Compressed Hemostatic Sponges: TCCC Guidelines Change 15-03.
---------------------------
Le recours à de petites éponges comprzsées et recouvertes de chitosan apparaît fortement séduisant à tel point que l'XStat fait partie du TCCC. Cependant les bases scientifiques qui pourraient permettre de recommander ce produit paraissent très faibles. La lecture attentive du document proposé permet de s'en rendre compte. D'une part une application sur un cochon dans des conditions de laboratoire par un expert ne reproduit pas les conditions de combat. Ce produit apparaît ne pass être adapté pour un emploi intra-thoracique, abdominal ou cervical et il est prévu pour une durée d'application de 04h, ce qui ne permet pas de couvrir un certain nombre de missions vraiment spéciales ou certaine régions comme le cou (1). On comprend bien dès lors toute la prudence à avoir avec ces produits que l'industrie nous présente comme "magique". Ils le sont peut être, et ceci concerne tous les pansements hémostatiques (2), mais il faut le prouver et ne pas céder aux sirènes effrénées du marketing des laboratoires. En la matière bien réaliser un packing de plaie, ne serait qu'avec une gaze standard doit rester un objectif fondamental, le reste étant un plus mais pas le fondamental.
---------------------------
Exsanguination from wounds in the so-called junctional regions of the body (i.e., the neck, the axilla, and the groin) was responsible for 19% of the combat fatalities who died from potentially survivable wounds sustained in Afghanistan or Iraq during 2001 to 2011. The development of improved techniques and technology to manage junctional hemorrhage has been identified in the past as a high-priority item by the Committee on Tactical Combat Casualty Care (CoTCCC) and the Army Surgeon General's Dismounted Complex Blast Injury (DCBI) Task Force. Additionally, prehospital care providers have had limited options with which to manage hemorrhage resulting from deep, narrow-track, penetrating trauma. XStat™ is a new product recently approved by the US Food and Drug Administration as a hemostatic adjunct to aid in the control of bleeding from junctional wounds in the groin or axilla. XStat has now been recommended by the CoTCCC as another tool for the combat medical provider to use in the management of junctional hemorrhage. The evidence that supports adding XStat to the TCCC Guidelines for the treatment of external hemorrhage is summarized in this paper.
01/11/2014
N'oublions pas le packing de plaie
A manikin model for study of wound-packing interventions to control out-of-hospital hemorrhage
Kragh JF et Al. Am J Emerg Med. 2014 Sep;32(9):1130-1
To the Editor,
With hemorrhage being the primary cause of mortality on the battlefield [1-3], wound-packing practice by US military medics in the wars since September 11, 2001, has changed from a conservative to an assertive approach. The foremost emphasis changed from preventing contamination to controlling hemorrhage. As no specific hemostatic dressings were available at the start of the wars, after such dressings were fielded, medics changed their approach by packing wounds with more gauze earlier in casualty care and deeper into subfascial cavitary wounds as a way to control hemorrhage. Although experienced medics and trainers favor an assertive approach, there is limited empirical evidence of improvements. In addition, with the development of various dressings with hemostatic properties [4-6], no systematic approach to trial wound-packing techniques easily has been developed. When a war ends and military medical care shifts toward peacetime duties and garrison work, skill sets in trauma care degrade as skill performance is less often. Furthermore, peacetime training reverts back toward everyday work such as sick call and away from future combat casualty care. The reversion tendency allows less training of new medics in combat casualty care than those who were trained during busy years of sustained combat; like nothing in peacetime, the present danger of combat during wartime focuses attention on hemorrhage control. A challenge for medics to be as well trained in peacetime in combat casualty care as during wartime is a recurring theme of military medicine.
Of the medical advances in prehospital combat casualty care during the current wars, we feel that the most important are regular tourniquets, junctional tourniquets, and wound packing because of their potential capacity to save numerous casualties from the most common cause of death on the battlefield—wound exsanguination. To not backslide on these 3 skills, we continue scholarly work to refine them. We call these skills the “Big 3,” and we have published mostly on tourniquets. To stimulate development of best practices in wound packing, we now focus the present report on an introductory test method to increase awareness of knowledge gaps within the science of wound packing.
The purpose of the present study is to introduce a laboratory model of hemorrhage with data comparing gauze wound packing and medical device use to better understand out-of-hospital hemorrhage control. In an approved protocol, we used a manikin model designed for the capacity to train medics in techniques of gauze wound packing for hemorrhage control in trauma care. The manikin trainer (Combat Ready Clamp [CRoC] Trainer Manikin; Operative Experience, Inc, North East, MD) had a gunshot wound of the right groin that bled water from the common femoral artery; the wound track went through the thigh posterior to anterior. We measured blood volume lost from bleeding, the application time, and hemorrhage control (yes-no). We had only 1 user who had never packed a wound prehospital and had never been trained in this task. We made 4 tests. The first test was that we used a type of gauze (QuikClot Combat Gauze; Z-Medica, Wallingford, CT) alone in accordance with its instructions for use (IFU) except we used no overwrap for pressure; the overwrap is the fourth and final step of the gauze IFU. The second test was like the first, but we used the full IFU that included use of an overwrap (AirWrap, RevMedx, Wilsonville, OR). The third test was only the A manikin model for study of wound-packing interventions to control out-of-hospital hemorrhage
use of the overwrap and no gauze; this test included no inflation of the pneumatic bladder within the overwrap. The fourth test included the gauze, the overwrap, and the inflation of the overwrap. Each test had 4 replicates.
The results showed an apparent differential performance of the methods of hemorrhage control, but the test order indicated possible learning that may be a confounder. The fourth test performed better than the first 3 with respect to reduced blood loss (Fig. 1), which may mean that the fourth method is best or that the user learned with experience. Perhaps both are true. Application time also improved (Fig. 2), and the evaluation was sensitive enough to detect longer application times with additional steps in the wound-wrapping process.
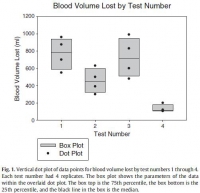
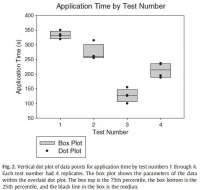 

There was no surprise that the gauze performed better as indicated by reduced blood loss (Fig. 1) when it was used fully in accordance with its IFU in that the second test performed better than the first. In addition, there was no surprise that the overwrap performed better when it was used fully in accordance with its intent in that the fourth test performed better than the third since the overwrap was designed to wrap over gauze.
The strength of this report is that it introduces a method of analyzing wound-packing techniques that generated hypotheses for testing. Hypothesis-driven experiments will follow this hypothesis generating report to check differential performance of techniques such as preliminarily studied here. The method permits learning curve analysis to see how fast users can be in hemorrhage control; we have previously found that tourniquet use, for example, appears to take more than 30 tests before users flatten their learning curve [7].
Determining optimal care techniques and training regimens may help to improve clinical performance. The limitation of the present report is its introductory design; the preliminary finding is only able to generate hypotheses. Future directions include analyses of techniques and learning curves.
John F. Kragh Jr., MD
US Army Institute of Surgical Research
Joint Base San Antonio
Fort Sam Houston, TX
Uniformed Services University of the Health Sciences
F. Edward Hébert School of Medicine Bethesda, MD
Corresponding author at: US Army Institute of Surgical Research
Damage Control Resuscitation, 3698 Chambers Pass
Ste B, Joint Base San Antonio
Fort Sam Houston, TX
E-mail address: john.f.kragh.civ@mail.mil
John Steinbaugh
RevMedx, Inc, Wilsonville, OR
Donald L. Parsons, PA-C
Combat Medic Training
US Army Medical Department Center and School
Joint Base San Antonio, Fort Sam Houston, TX
Robert L. Mabry, MD
Emergency Medical Services Fellowship
San Antonio Military Medical Center
Joint Base San Antonio
Fort Sam Houston, TX
Bijan S. Kheirabadi, PhD
Michael A. Dubick, PhD
Damage Control Resuscitation
US Army Institute of Surgical Research
Joint Base San Antonio
Fort Sam Houston, TX
| Tags : packing
14/10/2014
Pansements hémostatiques: Actualisation 2014 TCCC
Management of External Hemorrhage in Tactical Combat Casualty Care:
Chitosan-Based Hemostatic Gauze Dressings
TCCC Guidelines – Change 13-05
Le point sur l'emploi des pansements hémostatiques. Pas de grands changements le Quikclot Combat Gauze reste la référence mais le Chitosan et le Celox gauze sont considérés comme équivalents.
Cliquer sur le lien pour accéder à l'argumentaire
TCCC%20Bennett%20Hemostatic%20Drsngs%20TCCC%20Change%20JS...
03/09/2014
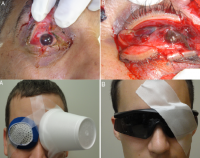
Ne comprimez pas les yeux
The use of rigid eye shields (Fox shields) at the point of injury for ocular trauma in Afghanistan
Mazolli RA et Al. J Trauma Acute Care Surg. 2014;77: S156-S162
METHODS:
RESULTS:
CONCLUSION:
23/04/2014
Le pansement OLAES MODULAR BANDAGE
Probablement le meilleur des pansements compressifs
C'est un pansement polyvalent qui permet trois actions. C'est d'abord un pansement plus compressif que le pansement israélien. Il permet en plus la réalisation d'un "packing" de plaie car il contient une bande de gaze en plus. Il permet également la réalisation d'un équivalent de pansement trois côtés ou un emballage succinct d'éviscération car il contient un petit champ transparent. Enfin de taille 6' au lieu de 4', il permet de couvrir des plaies plus importantes que le pansement israélien. Ce pansement est mis en place pour devenir le pansement standard de l'auxiliaire sanitaire.
Une démonstration de mise en oeuvre est --là-- . A noter qu'il existe une version non stérile, moins chère, en principe prévue pour l'entraînement.
06/11/2013
Pansement compressif: Un nouveau venu
Il existe une grande variété de pansements compressifs. L'armée française utilise le pansement dit israélien de la société Persys Medical et le pansement Olaes modular bandage de la société TacMed Solutions. En plus des bandes de compression, d'autres dispositifs on fait leur apparition visant à contrôler les hémorragies par une compression plus proximale (le CROC, le tourniquet abdominal, le SAMTourniquet, le Junctional emergency treatment tool). Ces dispositifs ont recours à des dispositifs de compression. L'Aiwrap est un pansement compressif proche des pansements en dotation auquel est adjoint une vessie qui une fois gonflée va majorer de manière importante la compression sous le pansement. Son positionnement dans une gamme de produit reste à préciser.
| Tags : hémorragie, pansement
05/11/2013
Pansement hémostatique: Utile?
Comparison of ChitoFlex®, CELOX™, and QuikClot® in control of hemorrhage
Devlin JJ et all. J Emerg Med. 2011 Sep;41(3):237-45
Un article qui complète le précédent et qui doit appeler à de la mesure sur l'emploi des pansements hémostatiques. Bien qu'il existe de nombreux travaux comparatifs prônant l'intérêt de tel ou tel dispositifs, le bien fondé de leur emploi extensif ne peut toujours pas être affirmé en condition de combat. Les messages pédagogiques doivent donc avant tout porter sur l'application correcte des pansements compressifs et le packing de plaie.
------------------------------------
BACKGROUND:
Exsanguinating extremity wounds remain the primary source of battlefield mortality. Operating forces employ three agents in Iraq: HemCon® (HemCon Medical Technologies, Inc., Portland, OR), QuikClot® (Z-Medica Corporation, Wallingford, CT), and CELOX™ (SAM Medical, Tualatin, OR). Anecdotal reports suggest that these agents are less useful on small entrance, linear-tract injuries. ChitoFlex® (HemCon Medical Technologies, Inc., Portland, OR) has been introduced but is untested.
STUDY OBJECTIVES:
To compare the equivalency of the ChitoFlex® dressing, QuikClot® ACS+™ dressing, CELOX™, and standard gauze in their effectiveness to control bleeding from non-cavitary groin wounds.
METHODS:
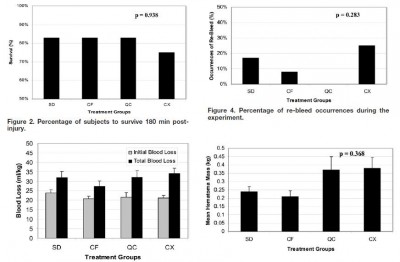
Forty-eight swine were randomly assigned to one of four treatment groups: standard gauze dressing (SD), ChitoFlex® dressing (CF), QuikClot® ACS+™ dressing (QC), and CELOX™ dressing (CX). A groin injury with limited vessel access was created in each animal. Subjects were resuscitated with 500 mL of hetastarch. The primary endpoint was 180-min survival. Secondary endpoints included total blood loss in mL/kg, incidence of re-bleeding, survival times among the animals that did not survive for 180 min, failure to achieve initial hemostasis, incidence of recurrent bleeding, time to initial re-bleeding, amount of re-bleeding, and mass of residual hematoma.
RESULTS:
Survival occurred in 10 of 12 SD animals, 10 of 12 CF animals, 10 of 12 QC animals, and 9 of 12 CX animals. No statistically significant difference was found.
CONCLUSION:
In our study of limited-access extremity bleeding, ChitoFlex® performed equally well in mitigating blood loss and promoting survival. The ChitoFlex® dressing is an equally effective alternative to currently available hemostatic agents. However, no agents were superior to standard gauze in our model of limited access.
| Tags : pansement
22/09/2013
Pansement hémostatique:Vraiment utile ?
Watters J et all. J Trauma. 2011;70:1413–1419
Un travail intéressant qui s'interroge sur le vrai intérêt des pansements hémostatiques. Sans remettre en question ce dernier cet article remet en question le positionnement de ce type de matériel. Dans un travail expérimental sur un modèle animal, un packing de plaie avec de la gaze simple serait plus rapide et tout aussi efficace qu'un packing effectué avec le Combat gauze ou le celox gauze.
-----------------------------------------------------------------
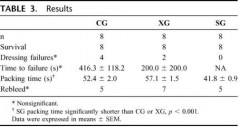
Results: All animals survived to study end. There were no differences in baseline physiologic or coagulation parameters or in dressing success rate (SG: 8/8, CG: 4/8, XG: 6/8) or blood loss between groups (SG: 260 mL, CG: 374 mL, XG: 204 mL; p > 0.3). SG (40 seconds ± 0.9 seconds) packed significantly faster than either the CG (52 ± 2.0) or XG (59 ± 1.9). At 120 minutes, all groups had a significantly shorter time to clot formation compared with baseline (p < 0.01). At 30 minutes, the XG animals had shorter time to clot compared with SG and CG animals (p < 0.05). All histology sections had mild intimal and medial edema. No inflammation, necrosis, or deposition of dressing particles in vessel walls was observed. No histologic or ultrastructural differences were found between the study dressings.
-----------------------------------------------------------------
"There are reasons that standard woven gauze bandages have existed for millennia. They are lightweight, absorbent, highly conformable, stable in a variety of environmental conditions, and inexpensive. Multiple advanced hemostatic agents have resulted in superior homeostasis, improved outcomes, and likely saved lives compared with SG when applied according to manufacturers’ recommendations for compression time. However, in a care under fire scenario or in a situation of mass casualties, compression times of 2 minutes to 5 minutes are not feasible. During ongoing battle, only lifethreatening injuries should be addressed and often the wounded must self-apply a tourniquet or dressing. An individual rendering self or buddy aid will need to continue to engage in battle as the first priority. Major vascular injuries, which cannot be controlled through application of a tourniquet, must be addressed as quickly as possible before profound bleeding incapacitates the casualty. Similarly, when there are persons with multiple injuries or wounds to treat, dressings must be rapidly placed and effective without prolonged hold times"
Conclusion: Ce qui compte c'est la compression et le packing de plaie
| Tags : pansement
17/07/2012
Pansement de plaie majeure du périné
07/05/2011
Biomatériaux pour le contrôle des hémorragies
Surtout pour les hémorragies compressibles.
Une revue très détaillée sur les biomatériaux utilisés pour cet usage. (ici)
21/11/2010
Pansements hémostatiques: Une vision US
Prehospital topical hemostatic agents – A review of the current literature
PHTLS Executive Council
Lance E. Stuke, M.D. MPH
Background: The 6th edition of the PHTLS textbook discusses three topical hemostatic agents which were approved by the U.S. Food and Drug Administration and available for prehospital use at the time: HemCon dressing, QuikClot, and TraumaDex. Data on these products was based primarily on anecdotal military reports and very little data was available on their use in the civilian prehospital setting. Several new products have arrived on the market after the release of the 6th edition of PHTLS and several important new studies have been published which will be reviewed here. The vast majority of these products have been researched and developed for use in the military setting in Iraq and Afghanistan although some limited civilian data is also available.
The perfect hemostatic dressing does not exist. Ideally, the dressing should be lightweight, easy to store, and able to be rapidly applied to a hemorrhaging wound. It should be conformable to the wound, allowing the hemostatic agent to reach areas of injury which are difficult to access with direct pressure (i.e. deep groin wounds). The dressing should cause minimal local tissue destruction, be easily removable from the wound, and not contain particles which can spread systemically. Finally, the dressing must not be washed away by rapid bleeding from high-flow blood vessels.
Manufacturers have tried various methods to deliver hemostatic agents into bleeding wounds. Some products are packaged into a granular form which can be poured directly into the wound. Others are incorporated into a dressing or mesh which allows the provider to apply direct pressure to the site of injury. This dressing can be formed either as a rigid bandage, a small bag, or a gauze which must be unrolled prior to application. Each method of preparation has distinct advantages and disadvantages depending on the location and type of injury being treated.
Literature and Product Review:
HemCon: HemCon dressing (Hemorrhage Control Technologies, Portland, OR), is composed of chitosan, a substance derived from arthropod skeletons. Chitosan dressings are thought to function by mechanically sealing the wound and adhering to surrounding tissue. HemCon is a dual-sided 4 x 4 inch rectangular bandage: a chitosan-containing active side which must be placed directly on the wound and a nonstick side which the provider uses to apply pressure. The efficacy of HemCon depends entirely on the bandage adhering well to the wound, which is difficult in wounds which aren’t flat and easily accessible. The bandage isn’t flexible and can break when forced into a wound. It is best applied to flat, superficial wounds which are easily accessible. HemCon has been studied in both the military and civilian settings. The military demonstrated a 97% success rate in controlling bleeding with HemCon.1,2 The civilian experience has been less optimistic, controlling bleeding in 27 of 34 cases studied (79%). Of the seven failures, six were felt to be due to user error, possibly due to less training by civilian EMS providers in the proper use of the product.3 An additional study using a complex groin injury model in swine noted an increase in the rate of rebleeding and mortality between those treated with HemCon versus QuikClot. The authors noted that application of HemCon was more difficult than other agents and all failures of HemCon were due to the bandage not adhering to the injured tissue to which it was applied.4
As previously noted, a disadvantage of the HemCon dressing is that it is relatively non-conformable and difficult to pack into deeper wounds. ChitoFlex is the latest development from HemCon Medical Technologies. It utilizes the same chitosan-based hemostatic agent but packages it into a gauze form. This allows the dressing to be packed into deep bleeding wounds for improved access to the site of hemorrhage. ChitoFlex is available in several sizes, including 1”x3”, 3”x9”, and as a 3”x28” roll. In one study, ChitoFlex was found to be equivalent, but not superior to QuikClot and Celox (a chitosan granule).5
WoundStat: WoundStat was an FDA-approved mineral-based agent consisting of granular smectite, a nonmetallic clay. When the granules were exposed to blood they absorbed water, swelled, and formed a clay paste with strong adhesiveness to the surrounding tissue. Initial studies were promising6,7,8 and it was used by the U.S. Army for a short time. However, later data demonstrated that the granules could cause injury to the blood vessels and make repair difficult. The granules were also shown to enter the circulatory system and cause thrombosis in distal organs.9 Because of these potentially serious side effects, the U.S. Army announced in April 2009 that WoundStat would no longer be used by their medical personnel.
QuikClot: QuikClot (Z-Medica, Wallingford, CT) is a granular product consisting of kaolin, which is a combination of inert minerals such as silicon, aluminum, magnesium, and sodium found in volcanic rock. When placed in a bleeding wound, it absorbs water thereby increasing the local concentration of clotting factors, platelets, and red blood cells to stimulate clot formation. A byproduct of its mechanism is a severe exothermic reaction, with heat generation of up to 70̊ C (158o F). This heat generation causes local tissue destruction and even burns. QuikClot has been studied in both the military and civilian sector, with up to 92% effectiveness in stopping hemorrhage.10 QuikClot was issued to U.S. soldiers in the Iraq and Afghanistan conflicts. Civilian use has been by a wide range of providers, including EMT/firefighters, paramedics, and police. Examples of civilian use include treatment of severe lacerations, gunshot wounds to the neck and even hemodialysis catheter dislodgement. Trauma surgeons have also used QuikClot for successful treatment of bleeding during surgery in the chest, abdomen, and pelvis. QuikClot was noted to have two significant weaknesses. Since it is a granular powder poured into a wound, it had limited usefulness in high-pressure bleeding (i.e. femoral artery bleed)as the granules were washed away by the bleeding before they were able to form a clot. Furthermore, the heat generated from its use was associated with several burns.
QuikClot production was stopped after development of several newer generation products. These newer generation products have minimal heat production and are packaged both as gauzes and in a bagged form. Currently Z-Medica sells QuikClot packaged in 2”x2” and 4”x4” gauze pads for use on superficial lacerations which don’t have severe bleeding. QuikClot has also developed a small kaolin-impregnated pad (QuikClot ACS+) and as a laparotomy pad (QuikClot Trauma Pad) for use by trauma surgeons in the operating room for cases of severe bleeding during surgery. This later product remains in the research phase and is not yet approved for widespread use.
QuikClot Combat GauzeTM is a 3”x4 yard long roll of nonwoven gauze impregnated with kaolin. Combat Gauze has all the advantages of normal gauze (easy application, flexible, large coverage area, and easily removable) with the additional advantage of hemostatic function from the kaolin. It is designed for packing into deep wounds which are actively bleeding (i.e. arterial injury in the groin). Prehospital personnel can also use combat gauze as they would any standard Kerlix gauze. Combat Gauze was recently compared to several newer generation products, including the HemCon RTS, and found to be superior and had no apparent side effects.11 Currently, QuikClot Combat Gauze is the only product endorsed by the Tactical Combat Casualty Care Committee and they recommend it as first line treatment for life-threatening hemorrhage on external wounds not amendable to direct pressure and tourniquet placement.
Summary:
- Numerous topical hemostatic products have been developed and released onto the market.
- Some of these products have since been discontinued, while others are widely used.
-Economic and medical considerations continue to make this a rapidly evolving and growing area of prehospital care. It is important for the EMS provider to remain cognizant of these products and their advantages, disadvantages, and complications as they continue to evolve.
PHTLS Recommendation: Topical hemostatic agents may be used to control hemorrhage occurring in sites not amenable to tourniquet placement and which cannot be controlled by direct pressure alone.
Bibliography
-
Achneck HE, Sileshi B, Jamiolkowski RM, et al. A comprehensive review of topical hemostatic agents: Efficacy and recommendations for use. Annals of Surgery 2010; 251: 217-228.
-
Mabry R and McManus JG. Prehospital advances in the management of severe penetrating trauma. Crit Care Med. 2008:36(7);S258-266.
-
Brown MA, Daya MR, Worley JA. Experience with chitosan dressings in a civilian EMS system. J Emerg Med. 2009;62:239-243.
-
Kozen BG, Kircher SJ, Henao J, et al. An alternative hemostatic dressing: comparison of CELOX, HemCon, and QuikClot.Acad Emerg Med. 2008; 15:74-81.
-
Devlin JJ, Kircher S, Kozen BG, et al. Comparison of ChitoFlex, CELOX, and QuikClot in control of hemorrhage. J Emerg Med. 2009 Apr 1 (Epub ahead of print).
-
Ward KR, Tiba MH, Holbert WH, et al. Comparison of a new hemostatic agent to current combat hemostatic agents in a swine model of lethal arterial hemorrhage. J Trauma. 2007;63:276-284.
-
Kheirabadi BS, Edens JW, Terrazas IB, et al. Comparison of new hemostatic granules/powders with currently deployed hemostatic products in a lethal model of extremioty arterial hemorrhage in swine. J Trauma. 2009;66:316-328.
-
Arnaud F, Parreno-Sadalan D, Tomori T, et al. Comparison of 10 hemostatic dressings in a groin transaction model in swine. J Trauma. 2009;67:848-855.
-
Bheirabadi BS, Mace JE, Terrazas IB, et al. Safety evaluation of new hemostatic agents, smectite granules, and kaolin-coated gauze in a vascular injury wound model in swine. J Trauma. 2010;68:269-278.
-
Rhee P, Brown C, Martin M, et al. QuikClot use in trauma for hemorrhage control: case series of 103 documented uses. J Trauma. 2008;64:1093-1099.
-
Kheirabadi BS, Scherer MR, Estep JS, et al. Determination of efficacy of new hemostatic dressings in a model of extremity arterial hemorrhage in swine. J Trauma. 2009;67:450-460.
| Tags : pansement
07/12/2008
Au sujet des pansements
| Tags : pansement