30/11/2019
Echo: Intérêt pas évident pour tous ?
Does point of care ultrasonography improve clinical outcomes in emergency department patients with undifferentiated hypotension? An international randomized controlled trial from the SHoC-ED investigators.
STUDY OBJECTIVE:
Point-of-care ultrasonography protocols are commonly used in the initial management of patients with undifferentiated hypotension in the emergency department (ED). There is little published evidence for any mortality benefit. We compare the effect of a point-of-care ultrasonography protocol versus standard care without point-of-care ultrasonography for survival and clinical outcomes.
METHODS:
This international, multicenter, randomized controlled trial recruited from 6 centers in North America and South Africa and included selected hypotensive patients (systolic blood pressure <100 mm Hg or shock index >1) randomized to early point-of-care ultrasonography plus standard care versus standard care without point-of-care ultrasonography. Diagnoses were recorded at 0 and 60 minutes. The primary outcome measure was survival to 30 days or hospital discharge. Secondary outcome measures included initial treatment and investigations, admissions, and length of stay.
RESULTS:
Follow-up was completed for 270 of 273 patients. The most common diagnosis in more than half the patients was occult sepsis. We found no important differences between groups for the primary outcome of survival (point-of-care ultrasonography group 104 of 136 patients versus standard care 102 of 134 patients; difference 0.35%; 95% binomial confidence interval [CI] -10.2% to 11.0%), survival in North America (point-of-care ultrasonography group 76 of 89 patients versus standard care 72 of 88 patients; difference 3.6%; CI -8.1% to 15.3%), and survival in South Africa (point-of-care ultrasonography group 28 of 47 patients versus standard care 30 of 46 patients; difference 5.6%; CI -15.2% to 26.0%). There were no important differences in rates of computed tomography (CT) scanning, inotrope or intravenous fluid use, and ICU or total length of stay.
CONCLUSION:
To our knowledge, this is the first randomized controlled trial to compare point-of-care ultrasonography to standard care without point-of-care ultrasonography in undifferentiated hypotensive ED patients. We did not find any benefits for survival, length of stay, rates of CT scanning, inotrope use, or fluid administration. The addition of a point-of-care ultrasonography protocol to standard care may not translate into a survival benefit in this group.
27/11/2019
SOF Sono
26/11/2019
TXA: Recommandé mais pas administré
An Analysis of Adherence to Tactical Combat Casualty Care Guidelines for the Administration of Tranexamic Acid.
BACKGROUND:
Hemorrhage is the leading cause of potentially survivable deaths in combat. Previous research demonstrated that tranexamic acid (TXA) administration decreased mortality among casualties. For casualties expected to receive a transfusion, the Committee on Tactical Combat Casualty Care (TCCC) recommends TXA. Despite this, the use and adherence of TXA in the military prehospital combat setting, in accordance with TCCC guidelines, is low.
OBJECTIVES:
We sought to analyze TXA administration and use among combat casualties reasonably expected to require blood transfusion, casualties with tourniquet placement, amputations, and gunshot wounds.
METHODS:
Based on TCCC guidelines, we measured proportions of patients receiving prehospital TXA: casualties undergoing tourniquet placement, casualties sustaining amputation proximal to the phalanges, patients sustaining gunshot wounds, and patients receiving ≥10 units of blood products within 24 h of injury. Univariable and multivariable analyses were also completed.
RESULTS:
Within our dataset, 255 subjects received TXA. Four thousand seventy-one subjects had a tourniquet placed, of whom 135 (3.3%) received prehospital TXA; 1899 subjects had an amputation proximal to the digit with 106 (5.6%) receiving prehospital TXA; and 6660 subjects had a gunshot wound with 88 (1.3%) receiving prehospital TXA. Of 4246 subjects who received ≥10 units of blood products within the first 24 h, 177 (4.2%) received prehospital TXA.
CONCLUSIONS:
We identified low TXA administration despite TCCC recommendations. Future studies should seek to both identify reasons for limited TXA administration and methods to increase future utilization.
20/11/2019
Reco Intubation vigile chez l'adulte
19/11/2019
Methoxyflurane: Très polluant ?
A Rigorous Evaluation of Methoxyflurane is Needed: Comment on “Methoxyflurane Versus Standard of Care for Acute Trauma-Related Pain in the Emergency Setting: Protocol for a Randomised, Controlled Study in Italy (MEDITA)”
--------------------------------
L'utilisation du Penthrox comme antalgique génère beaucoup de déchets (par ailleurs qui gaz à effets de serre), trop de déchets pour être recommandé? C'est du moins ce que pense une équipe nantaise.
--------------------------------
We have read with careful attention the article, “Methoxyflurane Versus Standard of Care for Acute Trauma-Related Pain in the Emergency Setting: Protocol for a Randomised, Controlled Study in Italy” by Fabbri et al. [1]. The authors present MEDITA (Methoxyflurane in Emergency Department in ITAly), a Phase IIIb, prospective, randomised, active-controlled, parallel-group, open-label, multicentre trial. We agree with the authors that there is a need for better evidence for the use of methoxyflurane in pain management in the emergency department. Low-dose methoxyflurane was approved based on the STOP! trial, which compared methoxyflurane only to placebo in young patients with acute trauma pain [2]. Despite a total absence of trials comparing methoxyflurane to an alternative analgesic available in the emergency department, huge efforts are made to introduce methoxyflurane to European emergency physicians, seen as a “global market” as reported in an online promotional video [3]. Indeed, they consider that methoxyflurane “can fit a very significant market need in terms of getting people out of pain, without them having to take a narcotic on board”.
We noticed that the authors will include patients with moderate pain (Numeric Rating Scale 4–6), and that in these patients, guidelines recommend an oral non opioid analgesic that can be given as soon as in the triage room [4]. Whilst the authors argue that the cost of a morphine treatment is high, we wonder if the authors will include in their analysis the cost and time of preparation and education of the patient for the use of the inhaler, and will compare this to the very cheap and straightforward administration of a pill. A cost-effectiveness analysis does not seem to be planned.
We also question the primary endpoint pain assessment at 10 min. The vast majority of the studies that evaluated pain management in the emergency department had a primary outcome at 30 min, as reported in the evidence-based review from Sin et al. [5]. Thus, the added value of satisfactory analgesia at 10 min versus 30 min is unclear, and may not be seen as clinically relevant. This highly question the long term effect of methoxyflurane and the need for other antalgic treatment after the single dose of methoxyflurane received.
Finally, in the era of global warming, and following the Katowice Climate Change Conference (24th session of the Conference of the Parties), we believe that we cannot promote a treatment that cause such amount of waste, as the hand-held inhaler must be throw out after usage. Emergency physicians must take an active part in protecting our planet and prescribe other more environment-friendly medications for pain management.
For all these reasons, we strongly believe that we need independent trials if we want to avoid another future medical reversal [6]. Two trials have been recently funded by the French ministry of health [7].
| Tags : me
Penthrox: Avis des canadiens
PLyo à température ambiante: Pas si sûr !
Freeze-dried plasma stability under prehospital field conditions.
BACKGROUND:
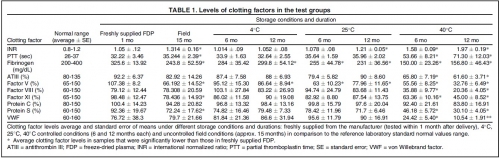
This study evaluated the effect of routine, uncontrolled, Israeli field storage conditions on the stability and efficacy of Lyo-Plas N freeze-dried plasma (FDP). We evaluated clotting factors V, VIII, and XI; proteins S and C; fibrinogen; partial thromboplastin time (PTT); antithrombin III (ATIII); von Willebrand factor (VWF); and international normalized ratio (INR) in FDP stored at 4°C, 25°C, and 40°C for 6 and 12 months, as well as FDP returned from field units after uncontrolled storage for 15 months (manufacturer's shelf life).
METHODS AND MATERIALS:
After reconstitution, clotting factor levels were compared to those of freshly supplied FDP doses.
RESULTS:
At 4°C for 12 months, factor V decreased slightly. At 25°C, average fibrinogen and factor V content were significantly lower at both periods, and INR was higher after 12 months. At 40°C, all samples were out of normal range in at least one clotting factor after 6 or 12 months. After field storage for 15 months, fibrinogen, factors V and XI, PTT, and protein S were significantly decreased, and INR increased.
However, these levels were still within laboratory norms. Statistically significant difference in clotting factors compared to laboratory normal range was found in INR (higher) and factor V (lower).
CONCLUSIONS:
Our data show minimal decreases in clotting factors in FDP after storage under field conditions, when compared to laboratory normal ranges. Along with the many advantages of FDP, this supports its use at the point of injury under battlefield conditions, despite uncontrolled storage environments. Under controlled storage conditions at 4°C, shelf life could possibly be extended, although further study is required.
04/11/2019
TCCC: Révision Août 2019