03/01/2020
IT Clamp: Pas si bien que cela ?
External Soft-Tissue Hemostatic Clamp Compared to a Compression Tourniquet as Primary Hemorrhage Control Device in Pilot Flow Model Study.
Acute blood loss represents a leading cause of death in both civilian and battlefield trauma, despite the prioritization of massive hemorrhage control by well-adopted trauma guidelines. Current Tactical Combat Casualty Care (TCCC) and Tactical Emergency Casualty Care (TECC) guidelines recommend the application of a tourniquet to treat life-threatening extremity hemorrhages. While extremely effective at controlling blood loss, the proper application of a tourniquet is associated with severe pain and could lead to transient loss of limb function impeding the ability to self-extricate or effectively employ weapons systems. As a potential alternative, Innovative Trauma Care (San Antonio, Texas USA) has developed an external soft-tissue hemostatic clamp that could potentially provide effective hemorrhage control without the aforementioned complications and loss of limb function. Thus, this study sought to investigate the effectiveness of blood loss control by an external soft-tissue hemostatic clamp versus a compression tourniquet.
HYPOTHESIS:
The external soft-tissue hemostatic clamp would be non-inferior at controlling intravascular fluid loss after damage to the femoral and popliteal arteries in a normotensive, coagulopathic, cadaveric lower-extremity flow model using an inert blood analogue, as compared to a compression tourniquet.
METHODS:
Using a fresh cadaveric model with simulated vascular flow, this study sought to compare the effectiveness of the external soft-tissue hemostatic clamp versus the compression tourniquet to control fluid loss in simulated trauma resulting in femoral and posterior tibial artery lacerations using a coagulopathic, normotensive, cadaveric-extremity flow model. A sample of 16 fresh, un-embalmed, human cadaver lower extremities was used in this randomized, balanced two-treatment, two-period, two-sequence, crossover design. Statistical significance of the treatment comparisons was assessed with paired t-tests. Results were expressed as the mean and standard deviation (SD).
RESULTS:
Mean intravascular fluid loss was increased from simulated arterial wounds with the external soft-tissue hemostatic clamp as compared to the compression tourniquet at the lower leg (119.8mL versus 15.9mL; P <.001) and in the thigh (103.1mL versus 5.2mL; P <.001).
CONCLUSION:
In this hemorrhagic, coagulopathic, cadaveric-extremity experimental flow model, the use of the external soft-tissue hemostatic clamp as a hasty hemostatic adjunct was associated with statistically significant greater fluid loss than with the use of the compression tourniquet.
02/01/2020
Le fibrinogène se conserve bien au chaud comme au froid
Stability of Fibrinogen Concentrate in Human Blood Samples: An In Vitro Study
Objectives:
This study was designed to assess the stability and functional activity of fibrinogen concentrates subjected to the changes in temperature and duration observed in field conditions.
Methods:
Fibrinogen concentrate was stored at -20°C (12 vials), 22°C (12 vials), and 50°C with 80% humidity (12 vials), for up to 6 mo. At each temperature, three vials of fibrinogen concentrate were taken out at 0, 1, 3, and 6 mo and reconstituted. On analysis days, blood samples were taken from a single healthy donor to collect plasma samples. The donor plasma was mixed with commercial fibrinogen-deficient plasma to make fibrinogen-adjusted plasma (FAP). An aliquot of the reconstituted fibrinogen concentrate was used for quantification of stored fibrinogen content (using STA-R) and function (Rotem - Fibtem) in FAP.
Results:
At 22°C for 0, 1, 3, and 6 mo, there were no significant changes observed in fibrinogen content (1,223 ± 42 mg/vial, 1,286 ± 86 mg/vial, 1,234 ± 76 mg/vial, and 1,178 ± 64 mg/vial), prothrombin time (13.5 ± 0.1 s, 13.7 ± 0.6 s, 13.3 ± 0.4 s, and 13.7 ± 0.2 s), or activated partial thromboplastin time (31.1 ± 0.2 s, 32.0 ± 0.2 s, 31.5 ± 0.2 s, and 32.0 ± 0.8 s), respectively. There were also no significant changes observed in any of the Fibtem measurements. Similarly, no differences were observed in these variables over time at -20°C and 50°C with 80% humidity.
Conclusions:
Fibrinogen concentrate maintained its content and function when stored at -20°C to 50°C with up to 80% humidity for 6 mo.
| Tags : fibrinogène
Methoxyflurane en altitude ? Mais oui
WIlkes M et Al. Wilderness Environ Med. 2018 Sep;29(3):388-391.
Methoxyflurane is a volatile, fluorinated anesthetic agent with analgesic properties. Although no longer used as an anesthetic due to concerns regarding renal toxicity in high doses, it has enjoyed a resurgence as an inhaled analgesic in prehospital care and in the emergency department. The agent is nonflammable and leads to rapid, titratable analgesia without intravenous access. The Penthrox inhaler device is light, robust, and straightforward to administer. Consequently, it has been proposed as an ideal analgesic for the remote high altitude setting.
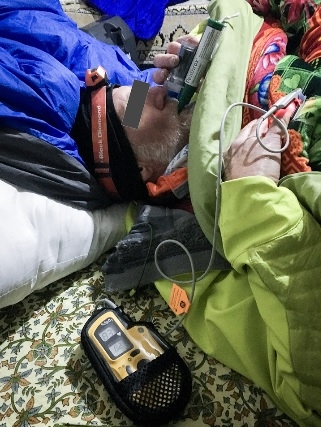
We report its use for procedural analgesia during suprapubic aspiration for acute urinary retention at a remote rescue post at night, in cold winter conditions, at 4470 m altitude in Machermo, Nepal. We found that methoxyflurane provided rapid, effective analgesia for our patient’s visceral and procedural pain. The inhaler was easy to administer, and the patient remained responsive to voice, with satisfactory oxygen saturation and respiratory rate throughout. We also briefly review the administration, dosing, efficacy, and safety of methoxyflurane and its role in remote medical care.
