02/12/2017
Fibrinogène en PREMIER pour le trauma
BACKGROUND:
Effective treatment of trauma-induced coagulopathy is important; however, the optimal therapy is still not known. We aimed to compare the efficacy of first-line therapy using fresh frozen plasma (FFP) or coagulation factor concentrates (CFC) for the reversal of trauma-induced coagulopathy, the arising transfusion requirements, and consequently the development of multiple organ failure.
METHODS:
This single-centre, parallel-group, open-label, randomised trial was done at the Level 1 Trauma Center in Innsbruck Medical University Hospital (Innsbruck, Austria). Patients with trauma aged 18-80 years, with an Injury Severity Score (ISS) greater than 15, bleeding signs, and plasmatic coagulopathy identified by abnormal fibrin polymerisation or prolonged coagulation time using rotational thromboelastometry (ROTEM) were eligible. Patients with injuries that were judged incompatible with survival, cardiopulmonary resuscitation on the scene, isolated brain injury, burn injury, avalanche injury, or prehospital coagulation therapy other than tranexamic acid were excluded. We used a computer-generated randomisation list, stratification for brain injury and ISS, and closed opaque envelopes to randomly allocate patients to treatment with FFP (15 mL/kg of bodyweight) or CFC (primarily fibrinogen concentrate [50 mg/kg of bodyweight]). Bleeding management began immediately after randomisation and continued until 24 h after admission to the intensive care unit. The primary clinical endpoint was multiple organ failure in the modified intention-to-treat population (excluding patients who discontinued treatment). Reversal of coagulopathy and need for massive transfusions were important secondary efficacy endpoints that were the reason for deciding the continuation or termination of the trial. This trial is registered with ClinicalTrials.gov, number NCT01545635.
FINDINGS:
Between March 3, 2012, and Feb 20, 2016, 100 out of 292 screened patients were included and randomly allocated to FFP (n=48) and CFC (n=52). Six patients (four in the FFP group and two in the CFC group) discontinued treatment because of overlooked exclusion criteria or a major protocol deviation with loss of follow-up. 44 patients in the FFP group and 50 patients in the CFC group were included in the final interim analysis.
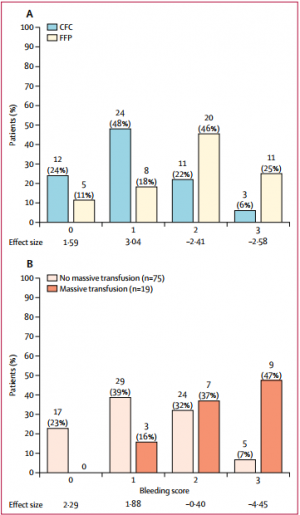
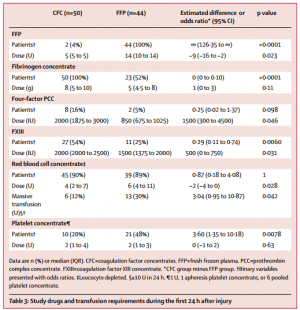
The study was terminated early for futility and safety reasons because of the high proportion of patients in the FFP group who required rescue therapy compared with those in the CFC group (23 [52%] in the FFP group vs two [4%] in the CFC group; odds ratio [OR] 25·34 [95% CI 5·47-240·03], p<0·0001) and increased needed for massive transfusion (13 [30%] in the FFP group vs six [12%] in the CFC group; OR 3·04 [0·95-10·87], p=0·042) in the FFP group. Multiple organ failure occurred in 29 (66%) patients in the FFP group and in 25 (50%) patients in the CFC group (OR 1·92 [95% CI 0·78-4·86], p=0·15).
INTERPRETATION:
Our results underline the importance of early and effective fibrinogen supplementation for severe clotting failure in multiple trauma. The available sample size in our study appears sufficient to make some conclusions that first-line CFC is superior to FFP.

Les commentaires sont fermés.